Answered step by step
Verified Expert Solution
Question
1 Approved Answer
From the following data solve using this equation: Notes: k= (solubility in H2O/solubility in DCM)= 6.0 (at 25C) V2 V2 +V,k) (Final mass of solute)H,0
From the following data solve using this equation:

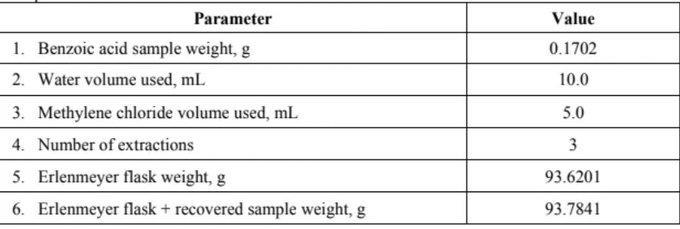
Notes: k= (solubility in H2O/solubility in DCM)= 6.0 (at 25C) V2 V2 +V,k) (Final mass of solute)H,0 (Initial mass of solute)H,0 Where: V= volume of organic solvent used per extraction V= original volume of water n= number of extractions k= distribution coefficient
Step by Step Solution
★★★★★
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Given that V 1 Volume of organic solvent used per extraction here it is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6369f3326505c_242611.pdf
180 KBs PDF File
6369f3326505c_242611.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


