Answered step by step
Verified Expert Solution
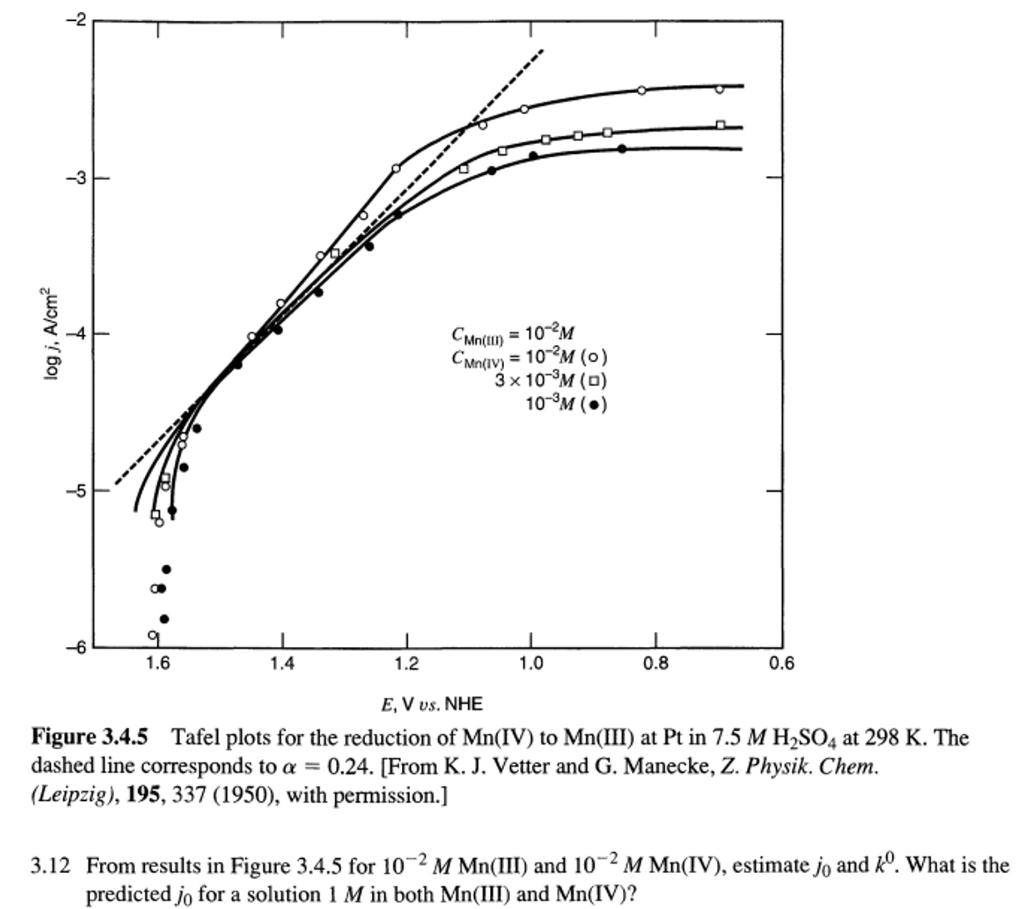
Question
1 Approved Answer
From the results below for 10 -2 M Mn(III) and 10 -2 M Mn(IV): 1) estimate j 0 and k 0 2) What is the
From the results below for 10-2 M Mn(III) and 10-2 M Mn(IV):
1) estimate j0 and k0
2) What is the predicted exchange current density j0 for a solution with 1M Mn(III) and 1M Mn(IV) inside? Explain the difference between the Tafel plot shown and an ideal Tafel plot at large overpotential (e.g. 1.0 - 0.6 V vs NHE). Hint: find out where is the equilibrium potential of Mn3+/4+ from the plot first. Note that the left bottom corner is neither the original point nor the equilibrium potential.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


