Answered step by step
Verified Expert Solution
Question
1 Approved Answer
From your IR spectrum explain what chemical bonds are present and absent in your unknown. Be specific. PLEASE ANSWER ALL QUESTIONS! Question one- three is
From your IR spectrum explain what chemical bonds are present and absent in your unknown.
Be specific. PLEASE ANSWER ALL QUESTIONS!
Question one- three is based on the 1HMNR spectra found from the chart ( on the last page) and explain. 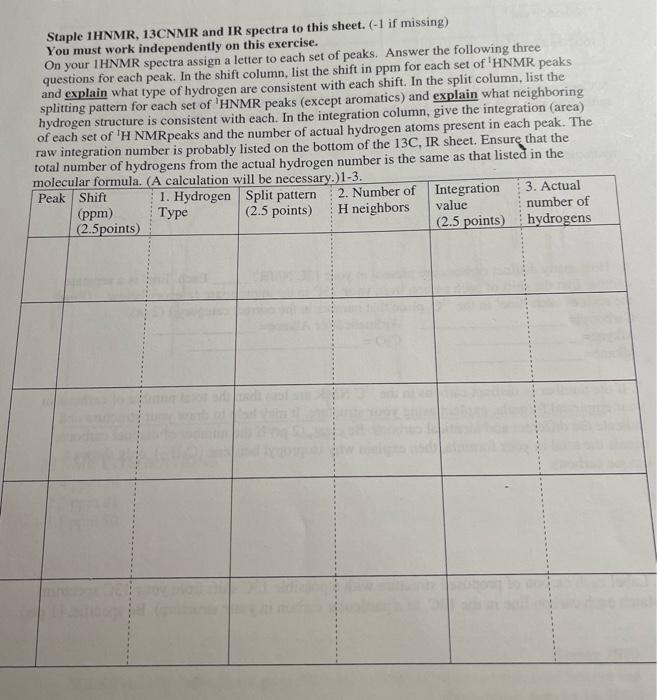 page 1
page 1 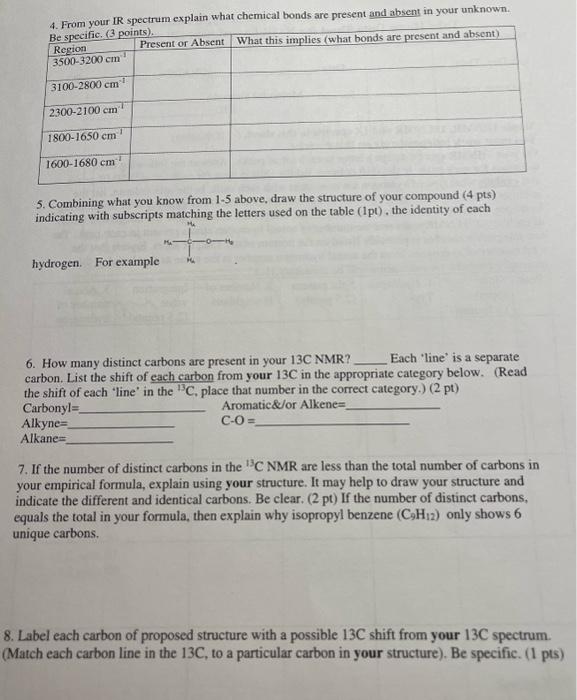 page 2
page 2 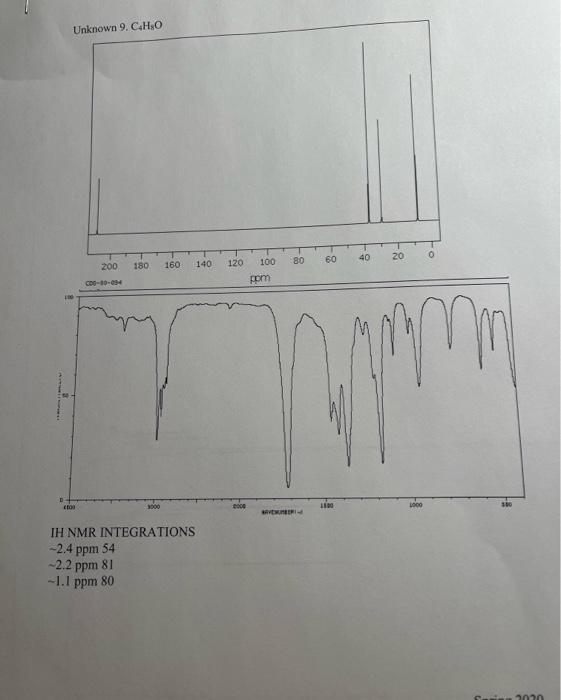
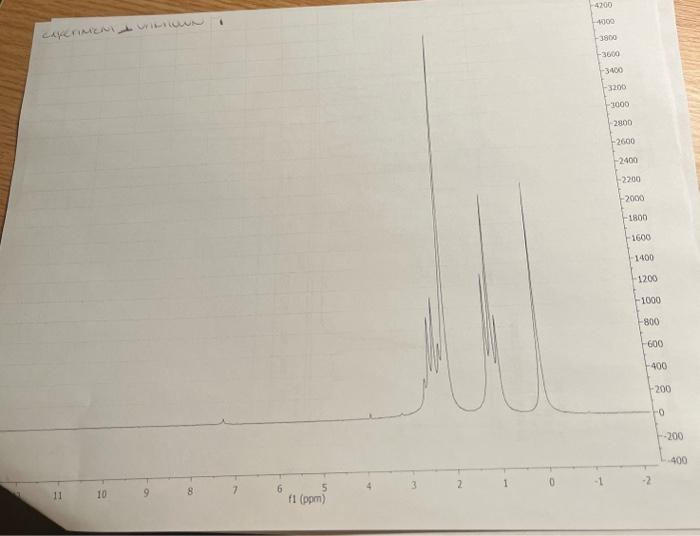
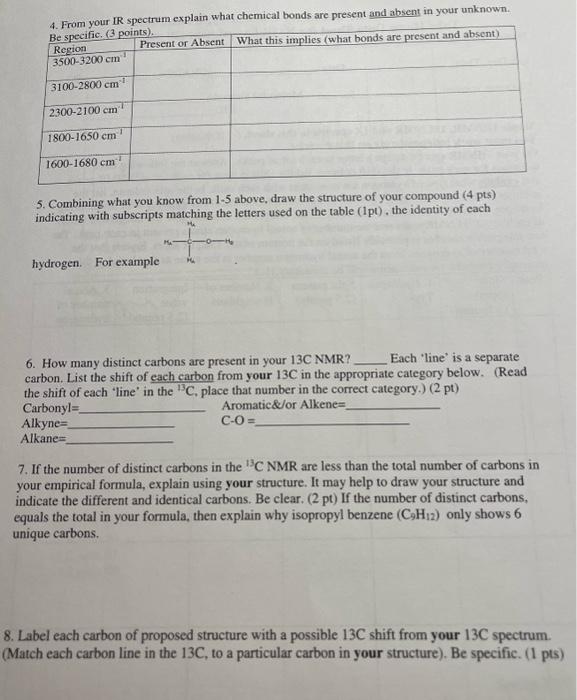
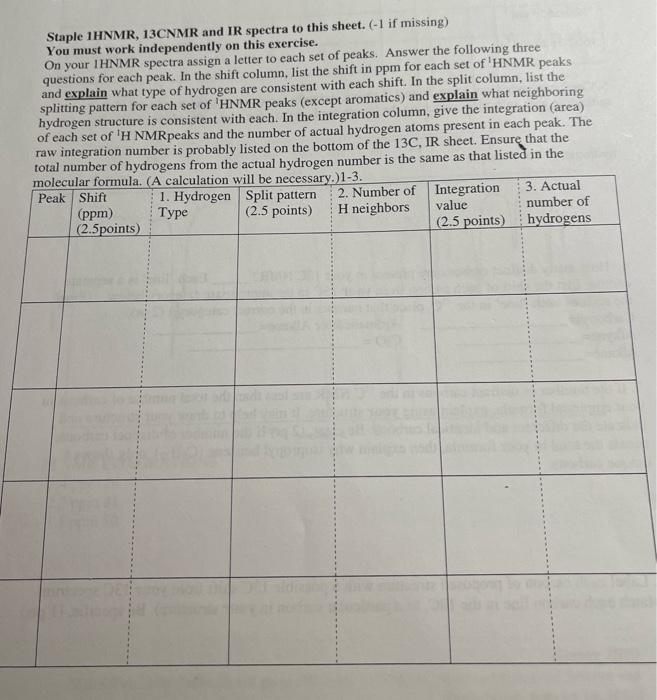
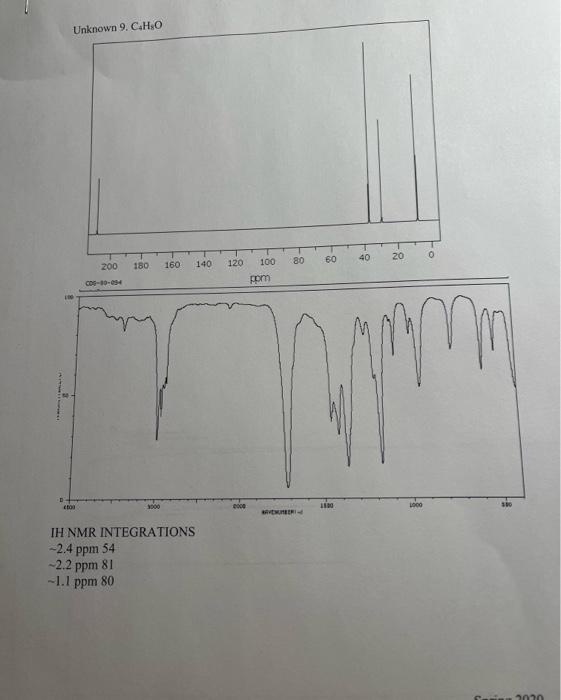
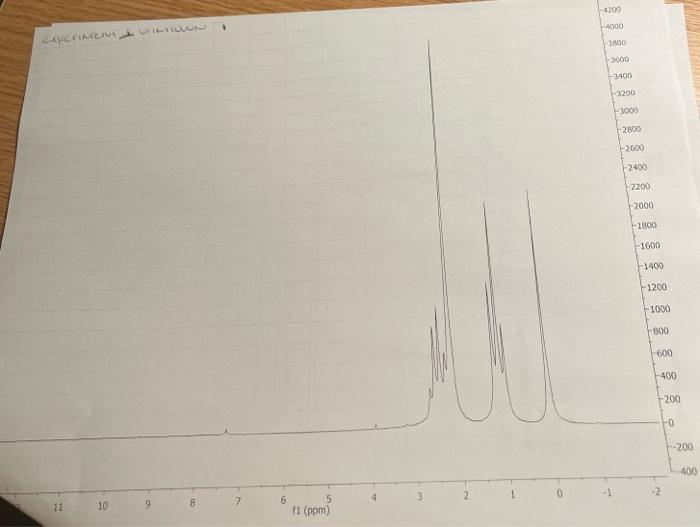
5. Combining what you know from 1-5 above, draw the structure of your compound ( 4 pts) indicating with subscripts matching the letters used on the table (1pt), the identity of each hydrogen. For exampli 6. How many distinct carbons are present in your 13C NMR? Each 'line' is a separate carbon. List the shift of each carbon from your 13C in the appropriate category below. (Read the shift of each 'line' in the 13C, place that number in the correct category.) (2 pt) Carbonyl= Aromatic\&/or Alkene = Alkyne = CO= Alkane= 7. If the number of distinct carbons in the 13C NMR are less than the total number of carbons in your empirical formula, explain using your structure. It may help to draw your structure and indicate the different and identical carbons. Be clear. ( 2pt ) If the number of distinct carbons, equals the total in your formula, then explain why isopropyl benzene (C9H12) only shows 6 unique carbons. 8. Label each carbon of proposed structure with a possible 13C shift from your 13C spectrum. (Match each carbon line in the 13C, to a particular carbon in your structure). Be specific. (1 pts) Staple 1HNMR, 13CNMR and IR spectra to this sheet. ( 1 if missing) You must work independently on this exercise. On your IHNMR spectra assign a letter to each set of peaks. Answer the following three questions for each peak. In the shift column, list the shift in ppm for each set of 'HNMR peaks and explain what type of hydrogen are consistent with each shift. In the split column, list the splitting pattern for each set of 'HNMR peaks (except aromatics) and explain what neighboring hydrogen structure is consistent with each. In the integration column, give the integration (area) of each set of 1H NMRpeaks and the number of actual hydrogen atoms present in each peak. The IH NMR INTEGRATIONS 2.4ppm54 2.2ppm81 1.1ppm80 cererimionl + untrious Staple 1HNMR, 13CNMR and IR spectra to this sheet. ( 1 if missing) You must work independently on this exercise. On your IHNMR spectra assign a letter to each set of peaks. Answer the following three questions for each peak. In the shift column, list the shift in ppm for each set of 'HNMR peaks and explain what type of hydrogen are consistent with each shift. In the split column, list the splitting pattern for each set of 'HNMR peaks (except aromatics) and explain what neighboring hydrogen structure is consistent with each. In the integration column, give the integration (area) of each set of 1H NMRpeaks and the number of actual hydrogen atoms present in each peak. The 5. Combining what you know from 1-5 above, draw the structure of your compound ( 4 pts) indicating with subscripts matching the letters used on the table (1pt), the identity of each hydrogen. For exampli 6. How many distinct carbons are present in your 13C NMR? Each 'line' is a separate carbon. List the shift of each carbon from your 13C in the appropriate category below. (Read the shift of each 'line' in the 13C, place that number in the correct category.) (2 pt) Carbonyl= Aromatic\&/or Alkene = Alkyne = CO= Alkane= 7. If the number of distinct carbons in the 13C NMR are less than the total number of carbons in your empirical formula, explain using your structure. It may help to draw your structure and indicate the different and identical carbons. Be clear. ( 2pt ) If the number of distinct carbons, equals the total in your formula, then explain why isopropyl benzene (C9H12) only shows 6 unique carbons. 8. Label each carbon of proposed structure with a possible 13C shift from your 13C spectrum. (Match each carbon line in the 13C, to a particular carbon in your structure). Be specific. (1 pts) IH NMR INTEGRATIONS 2.4ppm54 2.2ppm81 1.1 ppm 80  page 1
page 1  page 2
page 2 charts:
the unkown is at the top of the page


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


