Answered step by step
Verified Expert Solution
Question
1 Approved Answer
FTIR Spectrum of SO2 Use the data to answer the following questions 1. interpretation of the results, assessment of error, limitations of the method, and
FTIR Spectrum of SO2
Use the data to answer the following questions
1. interpretation of the results, assessment of error, limitations of the method, and suggestions for improvement.
2. Are the results expected? Why or why not? How do your results compare with known or literature values?
3. In addition, include in the discussion answers to any questions which were presented in the lab procedure itself
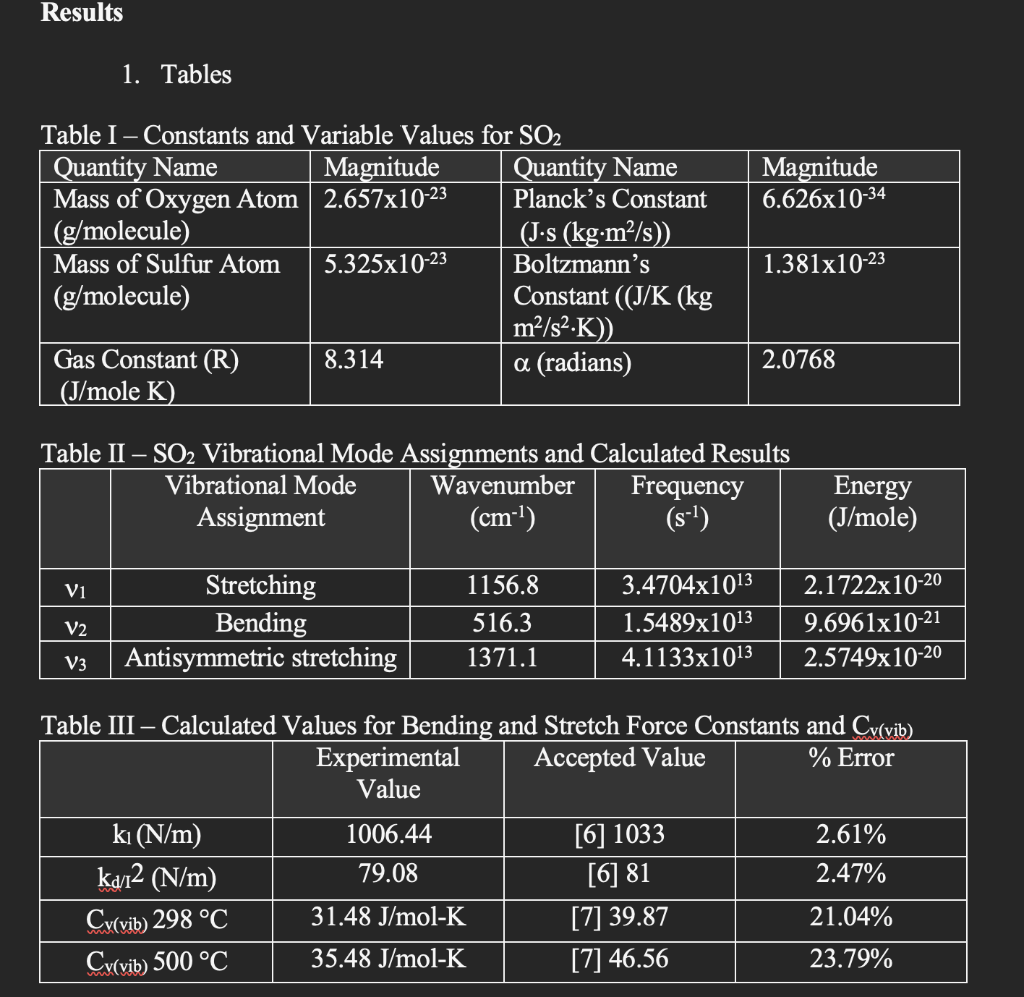
Results 1. Tables Magnitude 6.626x10-34 Table I - Constants and Variable Values for SO2 Quantity Name Magnitude Quantity Name Mass of Oxygen Atom 2.657x10-23 Planck's Constant (g/molecule) (J's (kgm2/s) Mass of Sulfur Atom 5.325x10-23 Boltzmann's (g/molecule) Constant ((J/K (kg m2/s2.K) Gas Constant (R) 8.314 a (radians) (J/mole K) 1.381x10-23 2.0768 Table II SO2 Vibrational Mode Assignments and Calculated Results Vibrational Mode Wavenumber Frequency Assignment (cm) (s-1) Energy (J/mole) Vi V2 Stretching Bending Antisymmetric stretching 1156.8 516.3 1371.1 3.4704x1013 1.5489x1013 4.1133x1013 2.1722x10-20 9.6961x10-21 2.5749x10-20 V3 Table III Calculated Values for Bending and Stretch Force Constants and Cy(vib) Experimental Accepted Value % Error Value ki (N/m) 1006.44 [6] 1033 2.61% 79.08 [6] 81 2.47% Cy(vib) 298 C 31.48 J/mol-K [7] 39.87 21.04% Cy(vib) 500 C 35.48 J/mol-K [7] 46.56 23.79% ka12 (N/m) Results 1. Tables Magnitude 6.626x10-34 Table I - Constants and Variable Values for SO2 Quantity Name Magnitude Quantity Name Mass of Oxygen Atom 2.657x10-23 Planck's Constant (g/molecule) (J's (kgm2/s) Mass of Sulfur Atom 5.325x10-23 Boltzmann's (g/molecule) Constant ((J/K (kg m2/s2.K) Gas Constant (R) 8.314 a (radians) (J/mole K) 1.381x10-23 2.0768 Table II SO2 Vibrational Mode Assignments and Calculated Results Vibrational Mode Wavenumber Frequency Assignment (cm) (s-1) Energy (J/mole) Vi V2 Stretching Bending Antisymmetric stretching 1156.8 516.3 1371.1 3.4704x1013 1.5489x1013 4.1133x1013 2.1722x10-20 9.6961x10-21 2.5749x10-20 V3 Table III Calculated Values for Bending and Stretch Force Constants and Cy(vib) Experimental Accepted Value % Error Value ki (N/m) 1006.44 [6] 1033 2.61% 79.08 [6] 81 2.47% Cy(vib) 298 C 31.48 J/mol-K [7] 39.87 21.04% Cy(vib) 500 C 35.48 J/mol-K [7] 46.56 23.79% ka12 (N/m)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


