Answered step by step
Verified Expert Solution
Question
1 Approved Answer
G. Use Table 1 to identify the metal used in the fishing sinker Data: Table 2 Temperature of Water and Waterflce mixture Table 3 Calorimetry
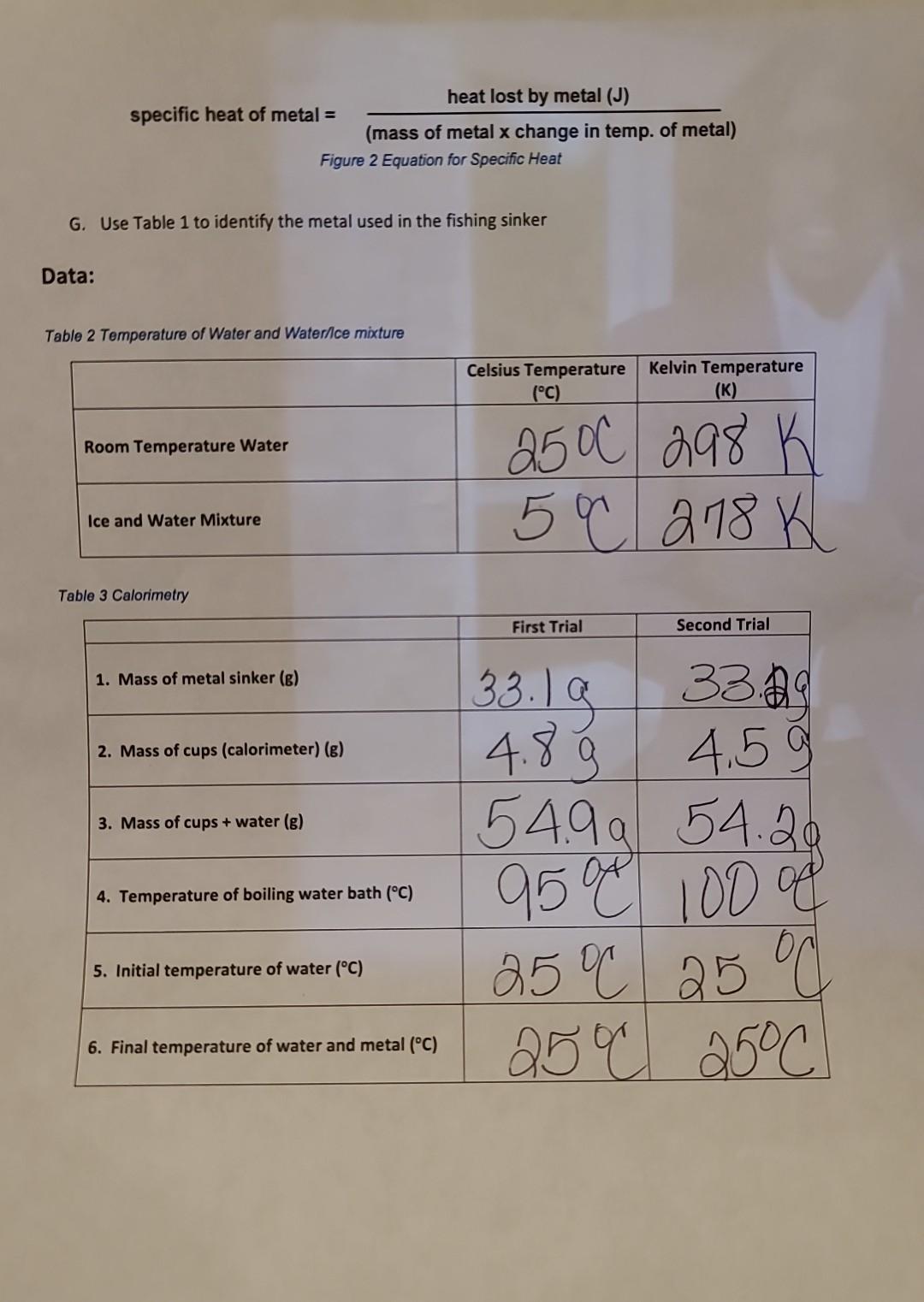
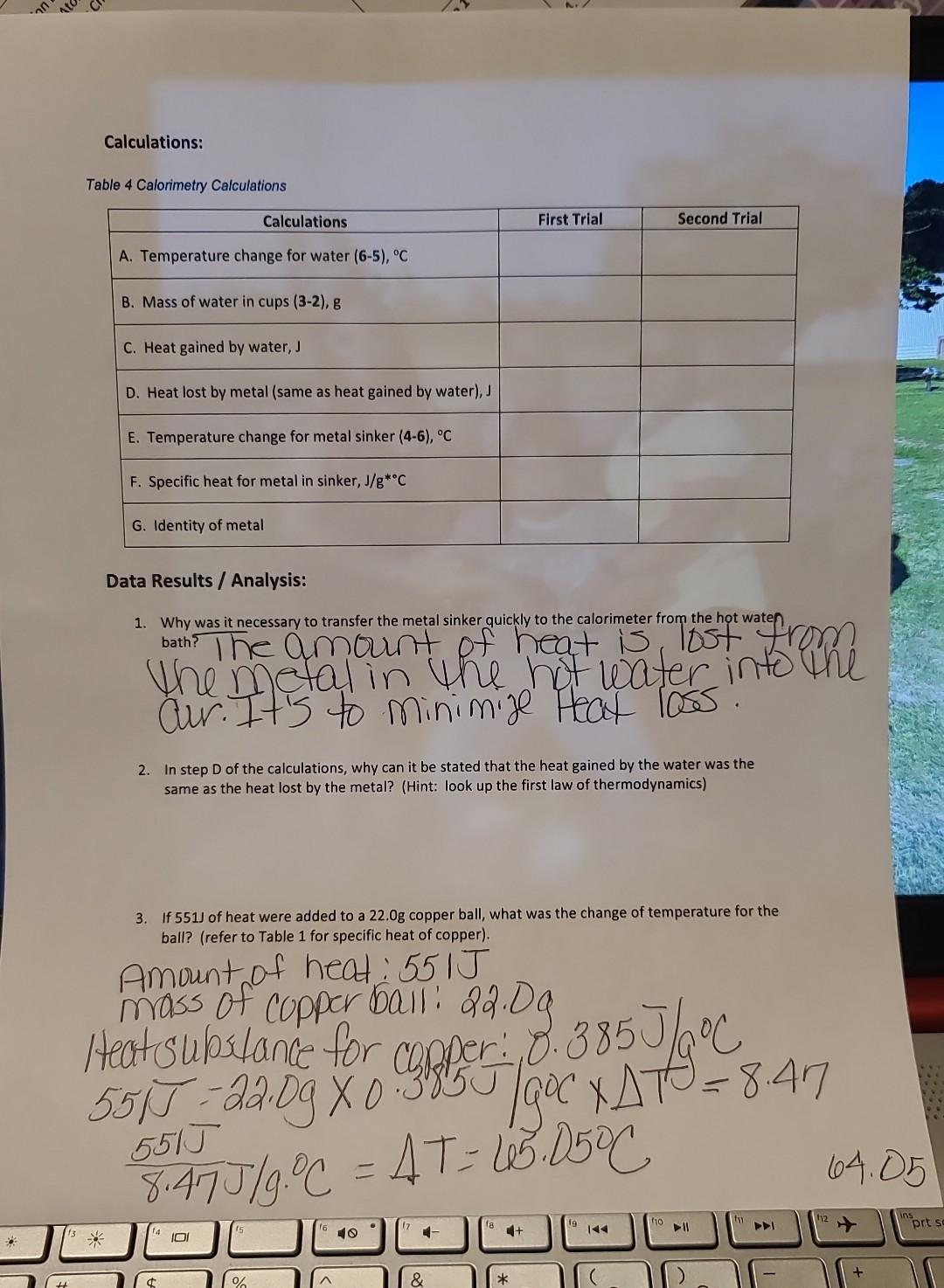
G. Use Table 1 to identify the metal used in the fishing sinker Data: Table 2 Temperature of Water and Waterflce mixture Table 3 Calorimetry Calculations: Table 4 Calorimetry Calculations Data Results / Analysis: 1. Why was it necessary to transfer the metal sinker quickly to the calorimeter from the hot waten 2. In step D of the calculations, why can it be stated that the heat gained by the water was the same as the heat lost by the metal? (Hint: look up the first law of thermodynamics) 3. If 551J of heat were added to a 22.0g copper ball, what was the change of temperature for the ball? (refer to Table 1 for specific heat of copper)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


