Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Gap -1 Pried 2 7 HE HI 3 LI 19 K 37 Rb 2 11 12 Na Mg P 98 98854 55 Co Be
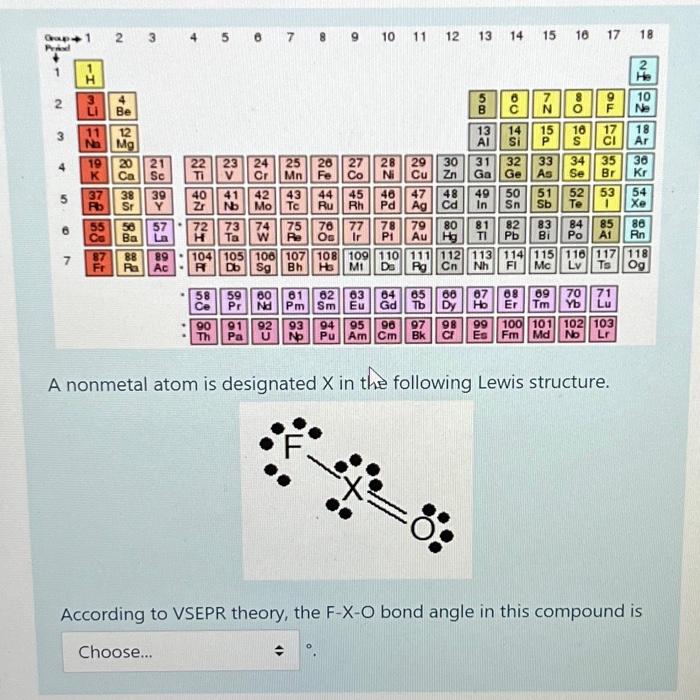
Gap -1 Pried 2 7 HE HI 3 LI 19 K 37 Rb 2 11 12 Na Mg P 98 98854 55 Co Be 4 5 6 7 21 22 23 24 Ca Sc NF ON NII 39 40 Y 41 Nb 57 72 73 74 75 76 77 Ba La Ta W Re Os 87 88 89 104 105 Fr Ra Ac Db 58 Ce 25 26 27 28 Cr Mn Fe Co Ni 90 Th 89 42 43 44 45 Mo Tc 59 60 Pr Nd 106 107 108 109 Sg Bh Ha MI 81 Pm 10 11 12 884223 Be 29 Cu 46 47 48 Ru Rh Pd Ag Cd 64 62 63 Sm Eu Gd 8588F25 30 65 Zn 78 79 PI Au Hg Tb 8680 66 13 14 15 56 22 58 98 13 Al 80 81 TI 49 6 C 14 Si 31 32 33 Ga Ge As In Sn 7 N 15 P 48: 50 51 16 8 110 111 112 113 114 115 116 117 De Rg Cn Nh FI Mc Lv Ts 17 18 5328-89CD TO Sb Te 16 17 18 S Ar 36 34 35 Se Br Kr 52 53 82 83 84 Pb Bi Po A1 85 91 92 93 94 95 96 97 Pa U No Pu Am Cm Bk 99 100 101 102 103 Es Fm Md No Lr A nonmetal atom is designated X in the following Lewis structure. 87 68 69 70 71 Ho Er Tm Yb Lu He 10 Ne 54 Xe 86 Rn 118 Og According to VSEPR theory, the F-X-O bond angle in this compound is Choose... Gap -1 Pried 2 7 HE HI 3 LI 19 K 37 Rb 2 11 12 Na Mg P 98 98854 55 Co Be 4 5 6 7 21 22 23 24 Ca Sc NF ON NII 39 40 Y 41 Nb 57 72 73 74 75 76 77 Ba La Ta W Re Os 87 88 89 104 105 Fr Ra Ac Db 58 Ce 25 26 27 28 Cr Mn Fe Co Ni 90 Th 89 106 107 108 109 Sg Bh Ha MI 59 60 Pr Nd 10 11 12 42 43 44 45 46 47 Ru Rh. Mo Tc 81 Pm 884223 Be 29 Cu 64 62 63 Sm Eu Gd 48 Pd Ag Cd 8588F25 30 65 Zn 78 79 PI Au Hg Tb 8680 66 13 14 15 56 22 58 98 13 Al 80 81 TI 49 6 C 14 Si 31 32 33 Ga Ge As In Sn 7 N 15 P 48: 50 51 16 8 110 111 112 113 114 115 116 117 De Rg Cn Nh FI Mc Lv Ts 17 18 5328-89CD TO Sb Te 16 17 18 S Ar 36 34 35 Se Br Kr 52 53 82 83 84 Pb Bi Po A1 85 91 92 93 94 95 96 97 Pa U No Pu Am Cm Bk 99 100 101 102 103 Es Fm Md No Lr A nonmetal atom is designated X in the following Lewis structure. 87 68 69 70 71 Ho Er Tm Yb Lu He 10 Ne 54 Xe 86 Rn 118 Og According to VSEPR theory, the F-X-O bond angle in this compound is Choose... Gap -1 Pried 2 7 HE HI 3 LI 19 K 2 11 12 Na Mg 37 Rb P 98 98854 55 Co Be 4 5 6 7 21 22 23 24 Ca Sc NF ON NII 41 42 43 44 45 46 Nb Mo Tc Ru Rh Pd 57 72 73 74 75 76 77 Ba La Ta W Re Os 87 88 89 104 105 Fr Ra Ac Db 39 40 Y 58 Ce 25 26 27 28 Cr Mn Fe Co Ni 90 Th 89 10 11 12 106 107 108 109 Sg Bh Ha MI 59 60 Pr Nd 81 Pm 78 PI 884223 Be 64 62 63 Sm Eu Gd 29 Cu 47 Ag 65 8588F25 Tb 30 91 92 93 94 95 96 97 Pa U No Pu Am Cm Bk 48 Zn Ga 13 14 15 56 22 58 8680 13 Al 66 31 80 81 Dy 98 49 Cd In Sn 79 82 83 Au Hg TI Pb Bi 6 C 14 Si 7 N 15 P 32 33 Ge As 50 51 48: 16 110 111 112 113 114 115 116 117 De Nh FI Mc Lv Ts Rg Cn 8 17 18 Sb Te 5328-89CD TO 16 17 18 S Ar 36 34 35 Br Kr Se 54 Xe 52 53 84 Po A1 85 87 68 69 70 71 Ho Er Tm Yb Lu 99 100 101 102 103 Es Fm Md No Lr A nonmetal atom is designated X in the following Lewis structure. He 10 Ne 86 Rn 118 Og According to VSEPR theory, the F-X-O bond angle in this compound is Choose...
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The Lewis structure you have provided shows a central atom X bonded to a fluorine F and an oxygen O ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


