Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You work for Xanadu, a luxury resort in the tropics. The daily temperature in the region is beautiful year-round, with a mean around 76
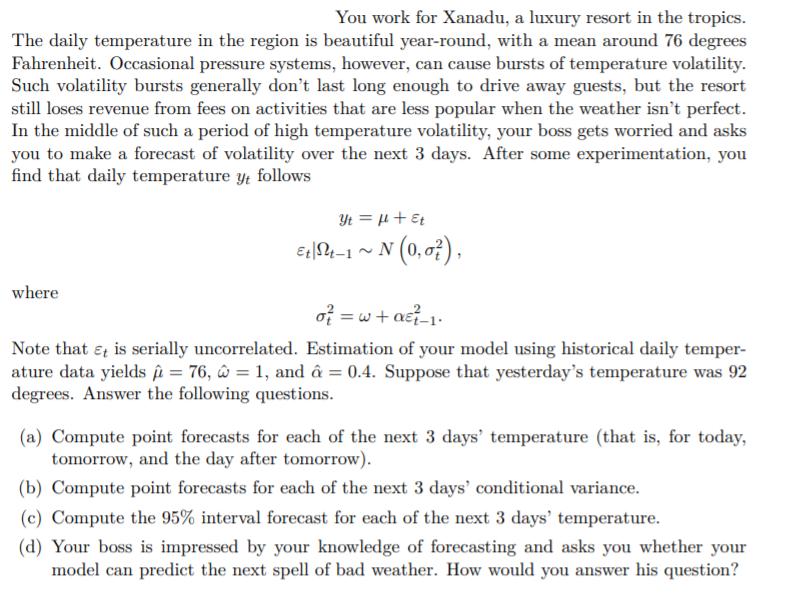
You work for Xanadu, a luxury resort in the tropics. The daily temperature in the region is beautiful year-round, with a mean around 76 degrees Fahrenheit. Occasional pressure systems, however, can cause bursts of temperature volatility. Such volatility bursts generally don't last long enough to drive away guests, but the resort still loses revenue from fees on activities that are less popular when the weather isn't perfect. In the middle of such a period of high temperature volatility, your boss gets worried and asks you to make a forecast of volatility over the next 3 days. After some experimentation, you find that daily temperature yt follows Yt = l + Et Et St-1 ~ N (0,0), where o = w+ a_1. Note that it is serially uncorrelated. Estimation of your model using historical daily temper- ature data yields = 76, = 1, and = 0.4. Suppose that yesterday's temperature was 92 degrees. Answer the following questions. (a) Compute point forecasts for each of the next 3 days' temperature (that is, for today, tomorrow, and the day after tomorrow). (b) Compute point forecasts for each of the next 3 days' conditional variance. (c) Compute the 95% interval forecast for each of the next 3 days' temperature. (d) Your boss is impressed by your knowledge of forecasting and asks you whether your model can predict the next spell of bad weather. How would you answer his question?
Step by Step Solution
★★★★★
3.53 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
ANSWERS a The point forecast for each of the next 3 days tempe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635da81b5c501_177723.pdf
180 KBs PDF File
635da81b5c501_177723.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


