Question
Gas (A) is dissolved in a liquid solvent (S) with a composition of 21 % by mole. The solvent is stripped to remove the
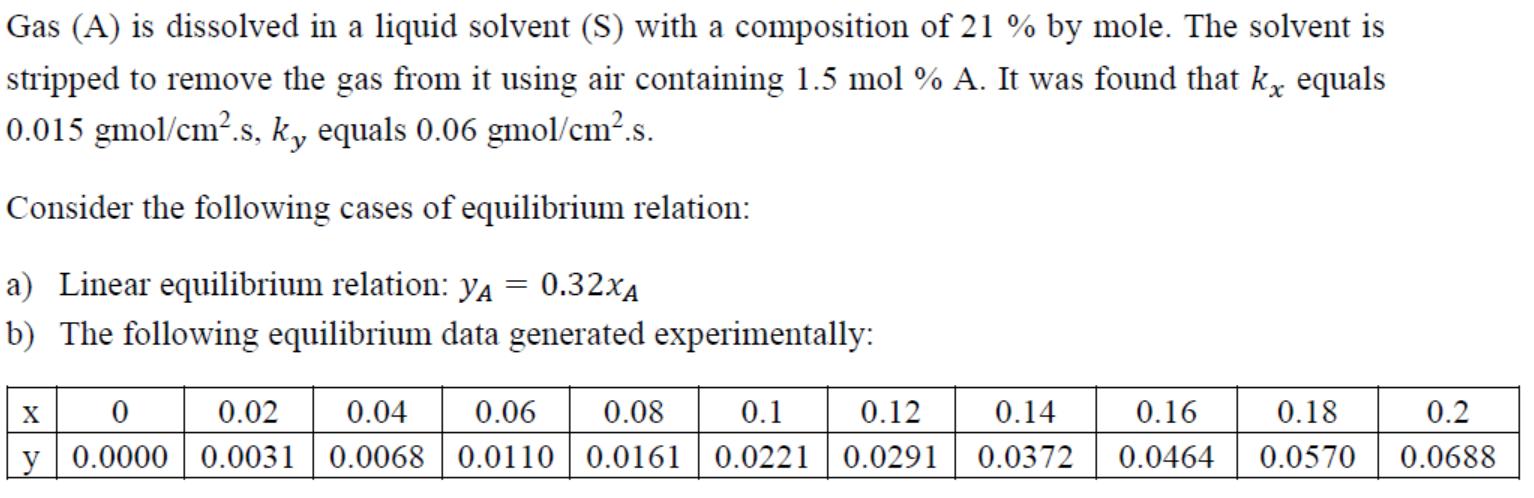

Gas (A) is dissolved in a liquid solvent (S) with a composition of 21 % by mole. The solvent is stripped to remove the gas from it using air containing 1.5 mol % A. It was found that k equals 0.015 gmol/cm.s, ky equals 0.06 gmol/cm.s. Consider the following cases of equilibrium relation: a) Linear equilibrium relation: YA 0.32XA b) The following equilibrium data generated experimentally: = X 0 0.02 0.04 0.06 0.08 y 0.0000 0.0031 0.0068 0.0110 0.0161 0.18 0.1 0.12 0.14 0.16 0.0221 0.0291 0.0372 0.0464 0.0570 0.2 0.0688 Calculate the following: 1. The interfacial compositions of A (XAi and Yai) 2. The molar flux of A. 3. The overall mass transfer coefficient Kx. 4. The contribution of the liquid phase resistance in the total mass transfer resistance.
Step by Step Solution
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Momentum Heat And Mass Transfer
Authors: James Welty, Gregory L. Rorrer, David G. Foster
6th Edition
1118947460, 978-1118947463
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



