Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Gas in a cylinder Vi A cylinder of ideal gas is shown above. The gas is sealed in the region below the piston, and
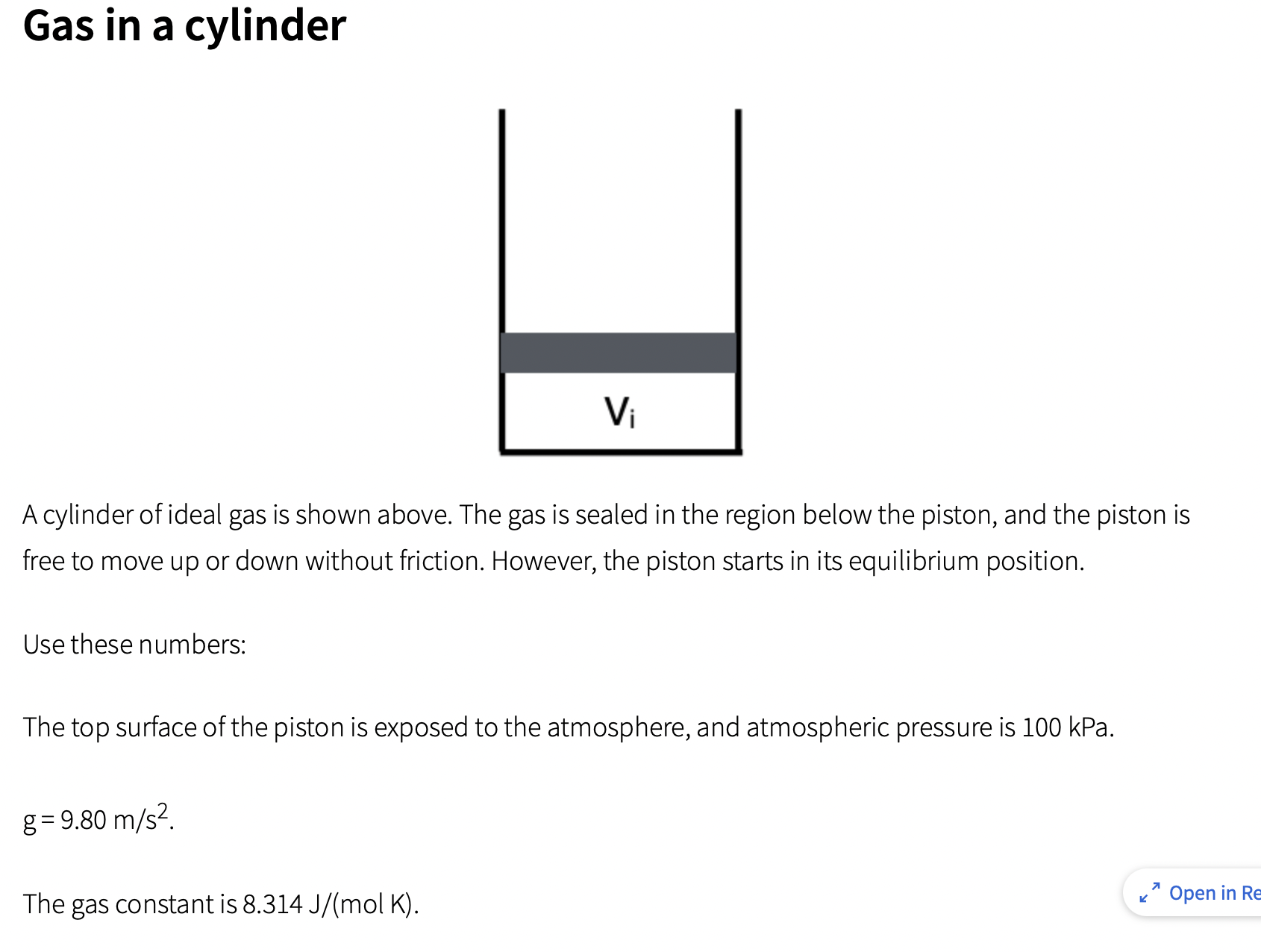
Gas in a cylinder Vi A cylinder of ideal gas is shown above. The gas is sealed in the region below the piston, and the piston is free to move up or down without friction. However, the piston starts in its equilibrium position. Use these numbers: The top surface of the piston is exposed to the atmosphere, and atmospheric pressure is 100 kPa. g= 9.80 m/s. The gas constant is 8.314 J/(mol K). K Open in Re The top surface of the piston is exposed to the atmosphere, and atmospheric pressure is 100 kPa. g= 9.80 m/s. The gas constant is 8.314 J/(mol K). The initial temperature is 25.0C. The cross-sectional area of the cylinder is 30.0 cm. That is also the area of the top surface of the piston and the bottom surface of the piston. The gas is an monatomic idea gas, so the change in internal energy is given by: AEint = nRAT := Part (a) Homework Answered Due Dec 5th, 12:15 PM The pressure inside the cylinder is initially 106 kPa, and the initial volume occupied by the gas is 1.60 L. Calculate the mass of the piston. kg Type your numeric answer and submit 324 7 Open in Part (b) Homework Unanswered Due Dec 5th, 12:15 PM Calculate the number of moles of ideal gas sealed in the cylinder. moles Type your numeric answer and submit Unanswered 5 attempts left Submit Part (c) Homework Unanswered Due Dec 5th, 12:15 PM Some heat is slowly added to the gas, so the gas expands at constant pressure to a final volume of 3.50 L. Calculate the work done by the gas inside the cylinder during this expansion. J Type your numeric answer and submit 7 Open in Reading Part (d) := Homework Unanswered Due Dec 5th, 12:15 PM Determine the final temperature of the gas, in Celsius. C Type your numeric answer and submit Unanswered 5 attempts left == Part (e) Homework Unanswered Due Dec 5th, 12:15 PM Determine how much heat was added to the gas. J Type your numeric answer and submit Submit + Open in Reading
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


