Question
GASTROCID is a commercial solt that may be composed of Na2CO3, NaHCO3and NaOH used as an ant acid medicine product... 4.55g of this sample
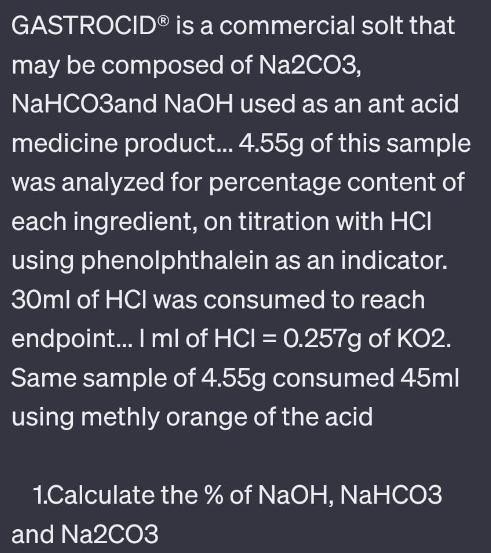
GASTROCID is a commercial solt that may be composed of Na2CO3, NaHCO3and NaOH used as an ant acid medicine product... 4.55g of this sample was analyzed for percentage content of each ingredient, on titration with HCI using phenolphthalein as an indicator. 30ml of HCl was consumed to reach endpoint... I ml of HCI = 0.257g of KO2. Same sample of 4.55g consumed 45ml using methly orange of the acid 1.Calculate the % of NaOH, NaHCO3 and Na2CO3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Database Systems Design Implementation and Management
Authors: Carlos Coronel, Steven Morris
11th edition
9781305323230, 1285196147, 1305323238, 978-1285196145
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



