Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Gen Chem 2 lab REPORT SUMMARY (10pts) Test for Silver lon From the procedure 7. Precipitate the Silver lon. Place 2 ml of the original
Gen Chem 2 lab 
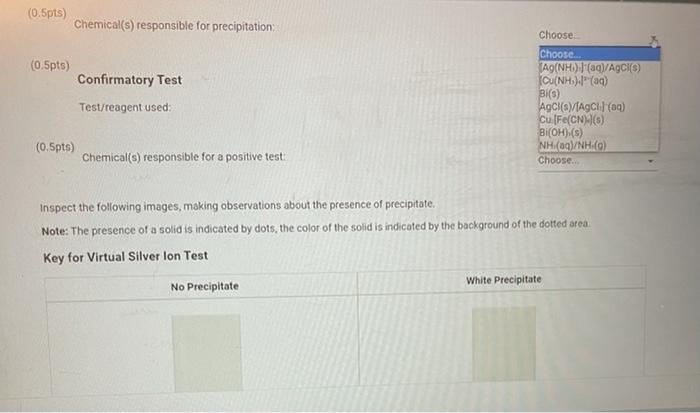
REPORT SUMMARY (10pts) Test for Silver lon From the procedure 7. Precipitate the Silver lon. Place 2 ml of the original reference solution in a small test tube, add 4-5 drops of 6.0 MHCI (Figure QC 3 in the lab manual) until no further evidence of precipitation occurs. Stir with a stirring rod and centrifuge the mixture. Test for complete precipitation by adding 1-2 additional drops of 6.0 M HCI. Ask your instructor about the safe operation of the centrifuge Decant and save the supernatant to test for the remaining cations (Step 9). Wash the precipitate with 5 drops of deionized water, centrifuge, and combine the supernatants 8. Confirmatory Test. Dissolve the precipitate with 6.0 M NH OH. Acidify the solution (check with litmus) with drops of 6.0M HNO3. A white precipitate confirms the presence of silver ion in the reference solution (0.5pts) Precipitation of Silver lon Test/reagent used (0.5pts) Chemical) responsible for precipitation Choose Choose nametest CH.COOH(aq) and K.Fe(CN)-() NH (80) NaOH (nam) Na Sn(OH)-(0) NH.OH(aq) and HNO (0) HCl(aq) Choose (0.5pts) Confirmatory Test Test/reagent used (10pts) Test for Silver lon From the procedure 7. Precipitate the Silver lon. Place - 2 mL of the original reference solution in a small test tube, add 4-5 drops of 6,0 M HCI (Figure QC.3 in the lab manual) until no further evidence of precipitation occurs. Stir with a stirring rod and centrifuge the mixture. Test for complete precipitation by adding 1-2 additional drops of 6.0 M HCI Ask your instructor about the safe operation of the centrifuge. Decant and save the supernatant to test for the remaining cations (Step 9). Wash the precipitate with 5 drops of deionized water, centrifuge, and combine the supernatants. B. Confirmatory Test. Dissolve the precipitate with 6.0 M NH OH. Acidify the solution (check with litmus) with drops of 6.0 M HNO3. A white precipitate confirms the presence of silver ion in the reference solution (0.5pts) Precipitation of Silver lon Test/reagent used Choose (0.5pts) Chemical(s) responsible for precipitation: (0.5pts) Confirmatory Test Choose... Choose [Ag(NH3)-1(aq)/Ag (3) [Cu(NH4).(aq) Bi(s) AQCISVIACI (aq) Cu.[Fe(CN).J(s) Bi(OH),(s) NH(aq)/NH (0) Test/reagent used: (0.5pts) Chemical(s) responsible for precipitation Choose (0.5pts) Confirmatory Test Test/reagent used Choose... A9(NH) Ia/Agciro [Cu(NH3)](aq) Bi(6) AGCIS)/AC: (0 Cu [Fe(CN) BI(OH),() NH(aq)/NH (0) Choose. (0.5pts) Chemical(s) responsible for a positive test: Inspect the following images, making observations about the presence of precipitate. Note: The presence of a solid is indicated by dots, the color of the solid is indicated by the background of the dotted area Key for Virtual Silver lon Test White Precipitate No Precipitate (0.5pts) Chemical(s) responsible for precipitation: Choose (0.5pts) Confirmatory Test Choose. Test/reagent used Choose flame test (0.5pts) CH.COOH(aq) and K [Fe(CN) Chemical(6) responsible for a positive test: NHMan) NaOH(mg) Na Sn(OH)-(0) NH.OH(aq) and HNO (4) Inspect the following images, making observations about the presence of precipitate. HCI (0) Note: The presence of a solid is indicated by dots, the color of the solid is indicated by the background of the dotted area. Key for Virtual Silver lon Test White Precipitate No Precipitate (0.5pts) Chemical(s) responsible for precipitation Choose (0.5pts) Confirmatory Test Test/reagent used: Choose (0.5pts) Chemical() responsible for a positive test: Choose Choose Ag(NH)."(aql/ACK) Inspect the following images, making observations about the presence of precipitate. [Cu(NH) || (94) 8 (6) Note: The presence of a solid is indicated by dots, the color of the solid is indicated by the background of the AgCl(s)[AgCl) () Cu[Fe(CN).-1) Key for Virtual Silver Ion Test B (OH) (6) NH.(gl/NH (0) No Precipitate White Precipitate 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


