Answered step by step
Verified Expert Solution
Question
1 Approved Answer
GENERAL BIOLOGY CHAPTER 2 QUESTIONS(please answer all) Refer to the figure below showing several structures of suoar mnloriilaes Which two structures represent a pair of
GENERAL BIOLOGY CHAPTER 2 QUESTIONS(please answer all) 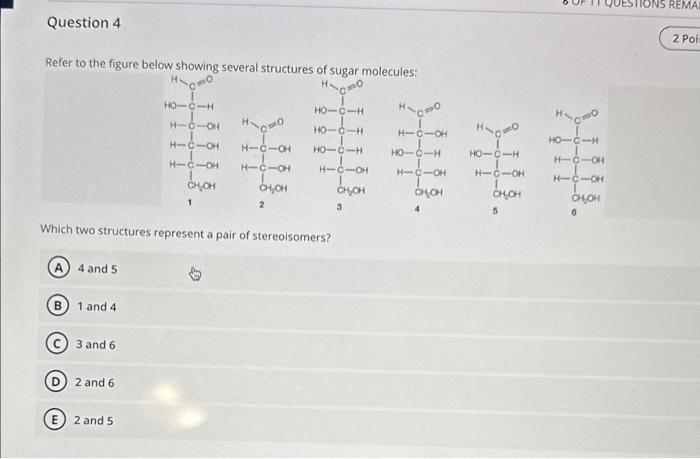

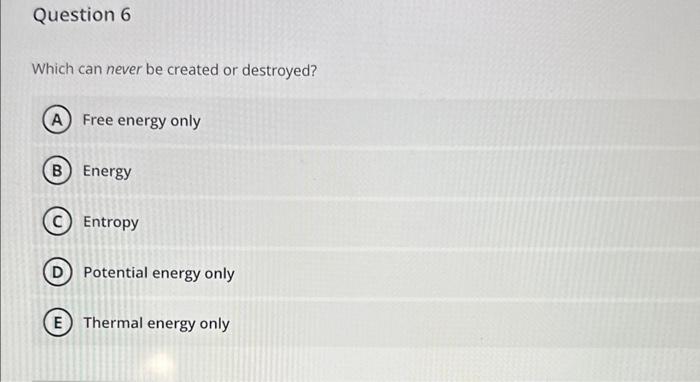

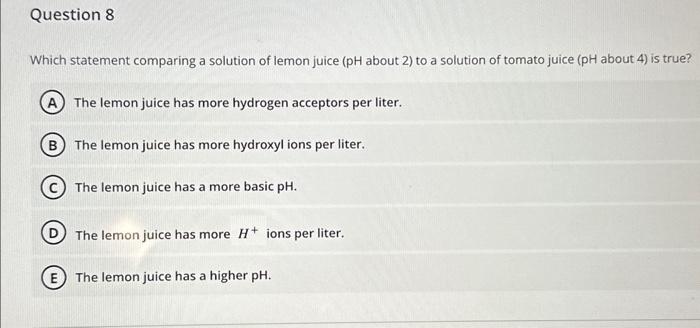

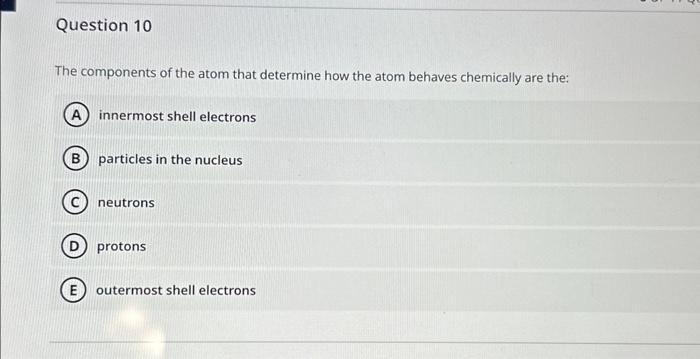

Refer to the figure below showing several structures of suoar mnloriilaes Which two structures represent a pair of stereoisomers? 4 and 5 1 and 4 3 and 6 2 and 6 2 and 5 The two covalent bonds in a water molecule are polar because: oxygen is less electronegative than hydrogen. water is a small molecule. oxygen is more electronegative than hydrogen. oxygen and hydrogen have similar electronegativities. (E) water is hydrophilic. Question 6 Which can never be created or destroyed? Free energy only Energy Entropy Potential energy only Thermal energy only Question 7 Hydrogens bonds form only between hydrogen and oxygen atoms within a molecule. form between two hydrogens atoms. involve a transfer of electrons. form weak interactions but can provide structural stability when many are found in a single molecule. form only between a weak electronegative atom and hydrogen. Which statement comparing a solution of lemon juice ( pH about 2) to a solution of tomato juice ( pH about 4) is true? The lemon juice has more hydrogen acceptors per liter. The lemon juice has more hydroxyl ions per liter. The lemon juice has a more basic pH. The lemon juice has more H+ions per liter. E The lemon juice has a higher pH. Glucose (C6H12O6) reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). How many molecules of carbon dioxide will be produced for each molecule of glucose that participates in this reaction? 2 1 3 (D) 6 (E) 12 The components of the atom that determine how the atom behaves chemically are the: innermost shell electrons particles in the nucleus neutrons protons outermost shell electrons Cholesterol is composed primarily of carbon and hydrogen atoms and is therefore: a base a buffer a polar molecule an acid insoluble in water 







Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


