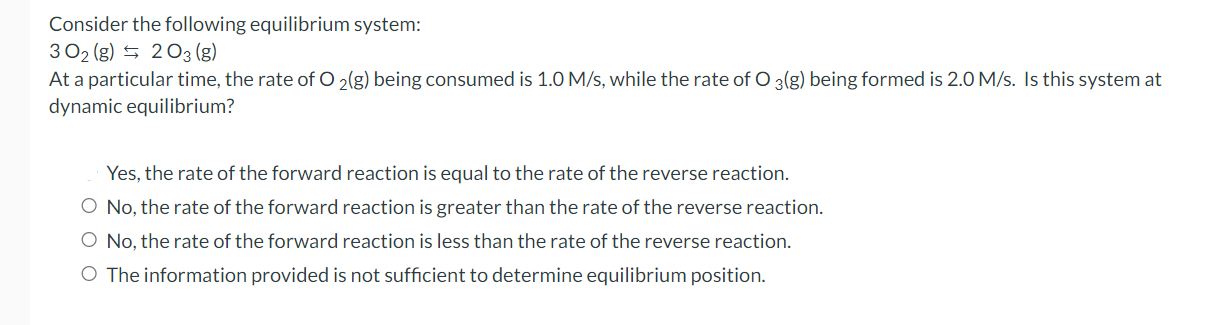
Question
General Chemistry for Engineers Lesson: Chemical Equilibrium - Dynamic Equilibrium in Chemical Systems - Equilibrium Laws - Equilibrium Laws for Heterogeneous Reactions - Position of
General Chemistry for Engineers
Lesson: Chemical Equilibrium - Dynamic Equilibrium in Chemical Systems - Equilibrium Laws - Equilibrium Laws for Heterogeneous Reactions - Position of Equilibrium and the Equilibrium Constant - Equilibrium and Le Chtelier's Principle - Using Equilibrium Constants to Calculate Concentrations
Directions: Answer the following problem by showing the complete solution. In return, I will give you a good rating. Thank you so much!
Note: Please be careful with the units and measurements in calculations. Thank you! Thank you so much!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


