Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given a 0.300 M solution of anserine at its isoelectric point and ready acess to 0.300M HCl, 0.300M NaOH and distilled water, describe the preparation
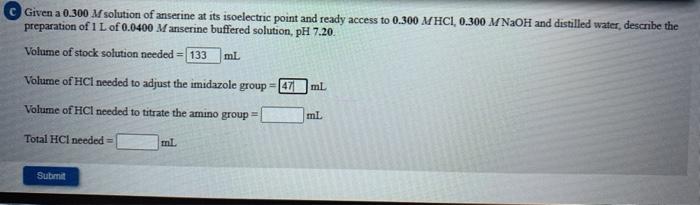
Given a 0.300 M solution of anserine at its isoelectric point and ready acess to 0.300M HCl, 0.300M NaOH and distilled water, describe the preparation of 1L of 0.0400M anserine buffered solution, ph 7.20.
Given a 0.300 M solution of anserine at its isoelectric point and ready access to 0.300 MHCI, 0.300 M NaOH and distilled water, describe the preparation of 1 L of 0.0400 Manserine buffered solution, pH 7.20. Volume of stock solution needed = 133 ml Volume of HCI needed to adjust the imidazole group-47 ml Volume of HCl needed to titrate the amino group = ml. Total HCl needed ml Submit Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


