Answered step by step
Verified Expert Solution
Question
1 Approved Answer
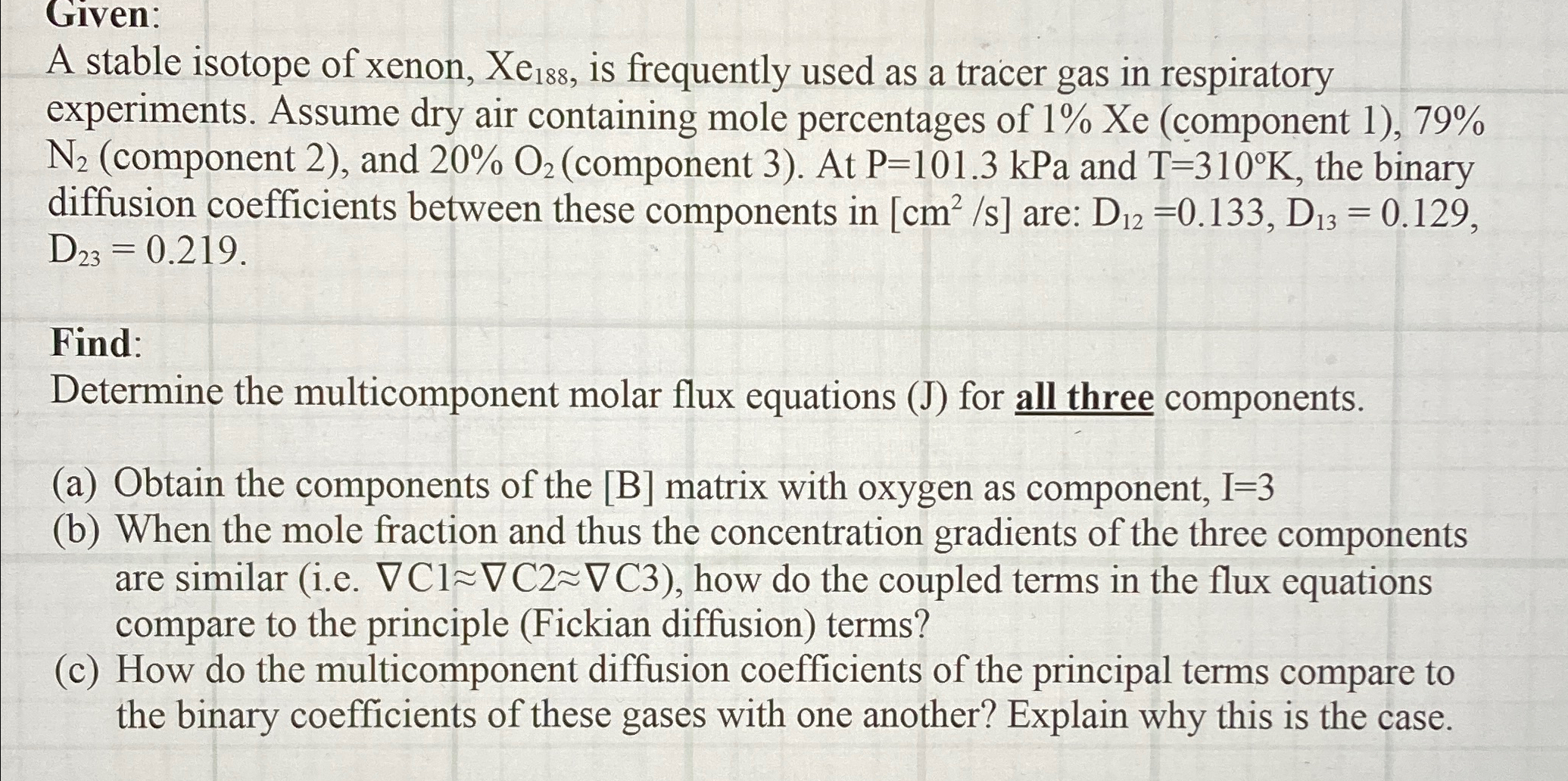
Given: A stable isotope of xenon, x e 1 8 8 , is frequently used as a tracer gas in respiratory experiments. Assume dry air
Given:
A stable isotope of xenon, is frequently used as a tracer gas in respiratory experiments. Assume dry air containing mole percentages of Xe component component and component At kPa and the binary diffusion coefficients between these components in are:
Find:
Determine the multicomponent molar flux equations for all three components.
a Obtain the components of the B matrix with oxygen as component,
b When the mole fraction and thus the concentration gradients of the three components are similar ie gradC~~gradC~~gradC how do the coupled terms in the flux equations compare to the principle Fickian diffusion terms?
c How do the multicomponent diffusion coefficients of the principal terms compare to the binary coefficients of these gases with one another? Explain why this is the case.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


