Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given below the temperature and composition data of an octane-toluene (O-T) mixture at 1 atm. in the table and the boiling points of octane
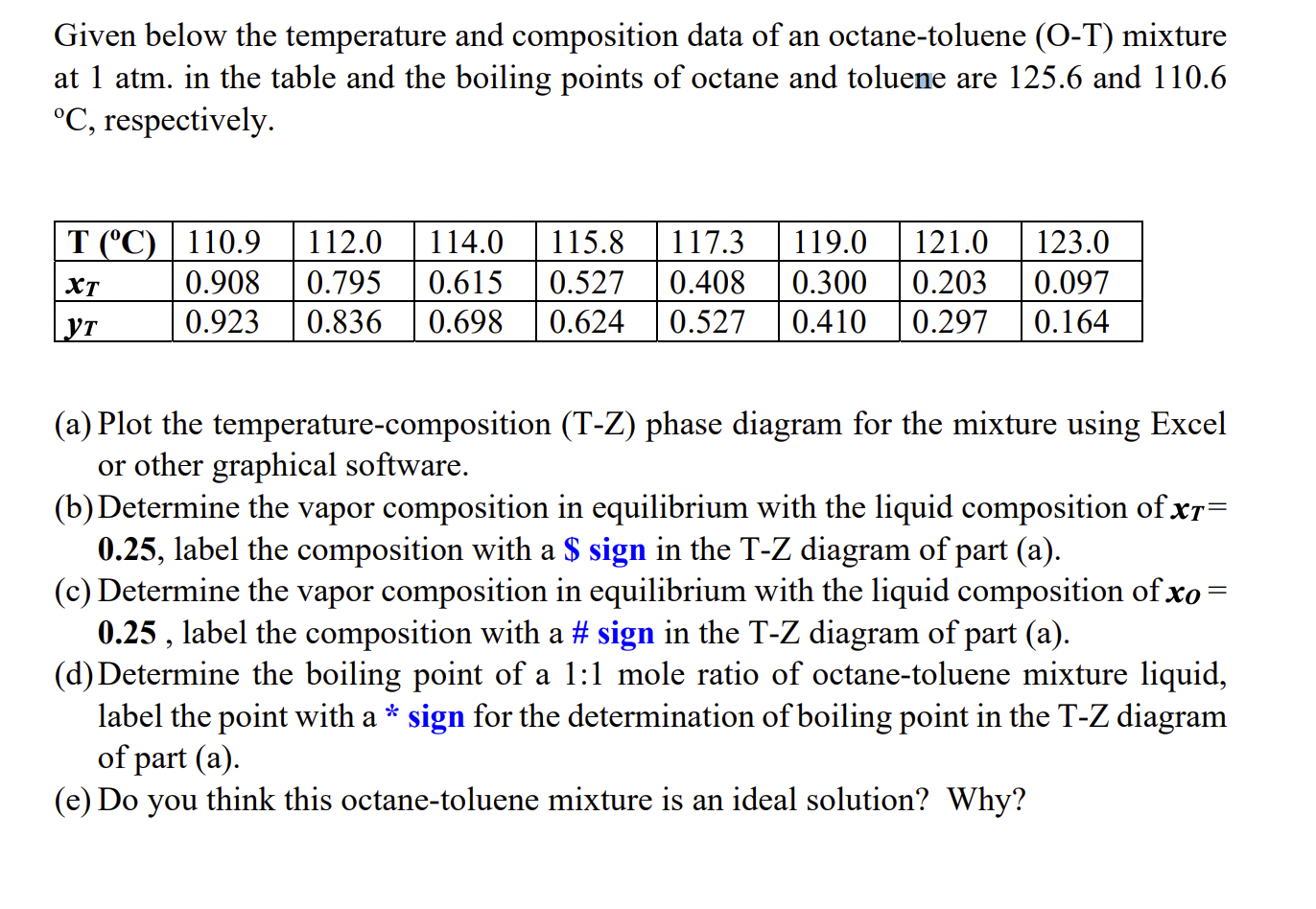
Given below the temperature and composition data of an octane-toluene (O-T) mixture at 1 atm. in the table and the boiling points of octane and toluene are 125.6 and 110.6 C, respectively. T (C) 110.9 112.0 114.0 115.8 117.3 119.0 121.0 123.0 0.908 0.795 0.615 0.527 0.408 0.300 0.203 0.097 0.923 0.836 0.698 0.624 0.527 0.410 0.297 0.164 XT YT (a) Plot the temperature-composition (T-Z) phase diagram for the mixture using Excel or other graphical software. (b) Determine the vapor composition in equilibrium with the liquid composition of x= 0.25, label the composition with a $ sign in the T-Z diagram of part (a). (c) Determine the vapor composition in equilibrium with the liquid composition of xo 0.25, label the composition with a # sign in the T-Z diagram of part (a). (d) Determine the boiling point of a 1:1 mole ratio of octane-toluene mixture liquid, label the point with a * sign for the determination of boiling point in the T-Z diagram of part (a). (e) Do you think this octane-toluene mixture is an ideal solution? Why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


