Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given; E G = -79.6127 Hartree, E T = -79.60806 Hartree, K B = The Boltzmann constant (1.3806x10 -23 J/K), and T= 300 K, what
Given; EG= -79.6127 Hartree, ET= -79.60806 Hartree, KB= The Boltzmann constant (1.3806x10-23J/K), and T= 300 K, what is the Boltzmann factor?
Use 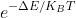
What have I done wrong here? The answer should be closer to 1 since the probability of ethane rotating around its bond is relatively high.

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


