Question: Given that, at 700 K, K, = 54.0 for the reaction H2(g) + I(g) = 2 HI(g) and K, = 1.04 X 10 for
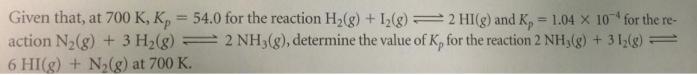
Given that, at 700 K, K, = 54.0 for the reaction H2(g) + I(g) = 2 HI(g) and K, = 1.04 X 10 for the re- action N,(g) + 3 H2(g) = 2 NH,(g), determine the value of K, for the reaction 2 NH,(g) + 31,(g) 6 HI(g) + N2(g) at 700 K. %3D !!
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


