Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the data in table 4.3 and the data tables, calculate the bond enthalpy and energy of the following: a.) C-H bond in CH4 b.)
Given the data in table 4.3 and the data tables, calculate the bond enthalpy and energy of the following: 
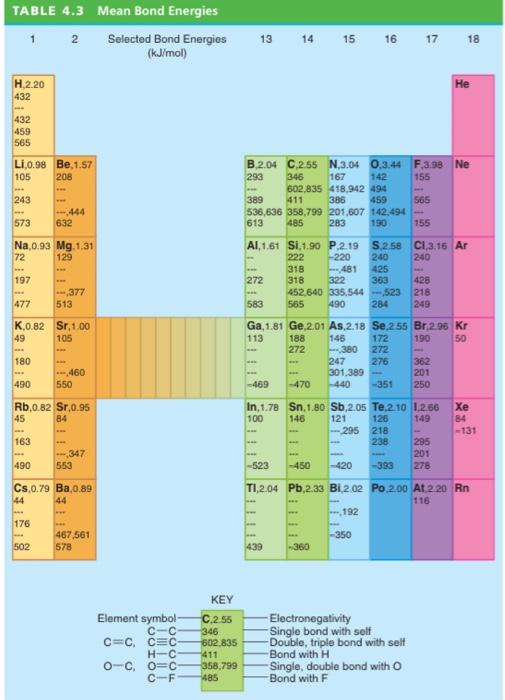
P4.7 Given the data in Table 4.3 and the data tables, calcu- late the bond enthalpy and energy of the following: a. The C-H bond in CH4 b. The CC single bond in CyH6 c. The C=C double bond in CH4 Use your result from part (a) to solve parts (b) and (c). c. TABLE 4.3 Mean Bond Energies 1 2 Selected Bond Energies (kJ/mol 13 14 15 16 17 18 He H.2.20 432 432 459 565 L1,0.98 Be.1.57 105 208 155 B.2.04 C.2.55 N,3.04 0.3.44 F.3.98 Ne 293 346 167 142 602,835 418,942 494 389 411 385 565 536,636 358,799 201,607 142.494 613 485 283 190 155 243 459 573 444 632 Na,0.93 Mg.1.31 72 129 197 AI 1.61 Si,1.90 P2.19 5.2.58 CI 3.16 Ar 222 -220 240 240 318 481 425 272 318 322 363 428 452.640 335,544 -523 218 583 565 490 284 249 -377 513 477 K.0.82 Sr.1.00 49 105 Ga 1.81 Ge 2.01 As,2.18 Se 255 Br.296 kr 113 188 146 172 190 50 272 272 247 276 362 301,389 201 -469 -470 -440 -351 250 -380 180 - 460 550 490 Xe Rb.0.82 Sr.0.95 45 84 84 -131 163 In, 1.78 Sn 1.80 Sb,2.05 Te, 2.10 1.256 100 146 121 126 149 - 295 218 238 285 201 -523 -450 -420 -393 278 - 347 553 490 C5,0.79 Ba, 0,89 44 T1,2.04 Pb,2.33 BI.2.02 Po 2.00 At 2 20 Rn 116 192 176 III -350 467,561 578 502 439 -360 KEY Element symbol CC C=CC=C HC O-CO=C CF C.2.55 346 602.835 411 358,799 485 Electronegativity Single bond with self Double, triple bond with self Bond with H Single, double bond with O Bond with F a.) C-H bond in CH4
b.) C-C single bond in C2H6
c.) C double bond C in C2H4
use your results from part a to solve parts b and c.
having a little trouble with this, would appreciate some help. please show work, thank you so much!


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


