Answered step by step
Verified Expert Solution
Question
1 Approved Answer
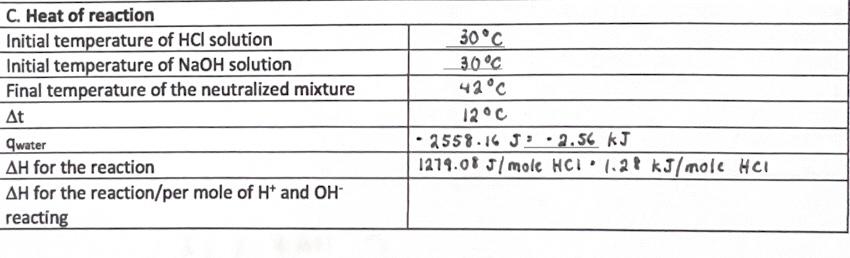
Given the following data, I need help with solving and understanding the last one. However, I am also quite unsure about my calculations for q
Given the following data, I need help with solving and understanding the last one. However, I am also quite unsure about my calculations for q water and delta H for the reaction. I would really appreciate it if I can receive some help verifying it and/or commenting on whether I am on the right track or not. Thank you!

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


