Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the following free energy diagram for oxygen evolution reaction a 6 b 0.0 5 Ideal OER catalyst O2(g) Real OER catalyst RuO Iro,
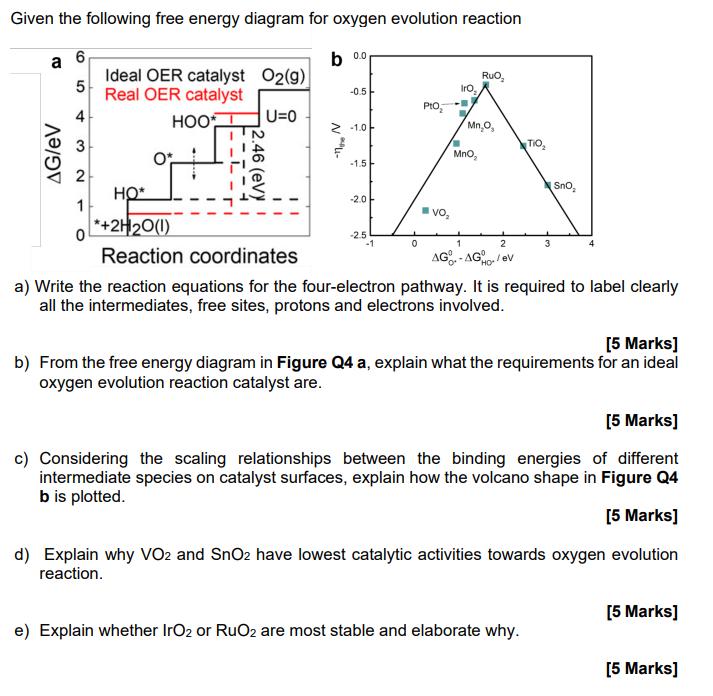
Given the following free energy diagram for oxygen evolution reaction a 6 b 0.0 5 Ideal OER catalyst O2(g) Real OER catalyst RuO Iro, -0.5 PtO AG/EV + 3 2 HOOT U=0 -1.0 Mn,O, .46 TIO 4- MnO, -1.5 SnO 1 HO* *+2H20(1) Reaction coordinates -2.0 Vo -2.5 -1 0 1 2 3 4 AG-AG/ev HO a) Write the reaction equations for the four-electron pathway. It is required to label clearly all the intermediates, free sites, protons and electrons involved. [5 Marks] b) From the free energy diagram in Figure Q4 a, explain what the requirements for an ideal oxygen evolution reaction catalyst are. [5 Marks] c) Considering the scaling relationships between the binding energies of different intermediate species on catalyst surfaces, explain how the volcano shape in Figure Q4 b is plotted. [5 Marks] d) Explain why VO2 and SnO2 have lowest catalytic activities towards oxygen evolution reaction. [5 Marks] e) Explain whether IrO2 or RuO2 are most stable and elaborate why. [5 Marks]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Reaction equations for the fourelectron pathway The fourelectron pathway for oxygen evolution often occurring at the anode during water electrolysis or photosynthesis can be described by the followi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


