Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the following reaction .23 Fe(OH)3 + e- + 3H+ Fe+2 + 3 H20 -166.5 A -18.85 -56.68 the Pe of this reaction (2) 2.40

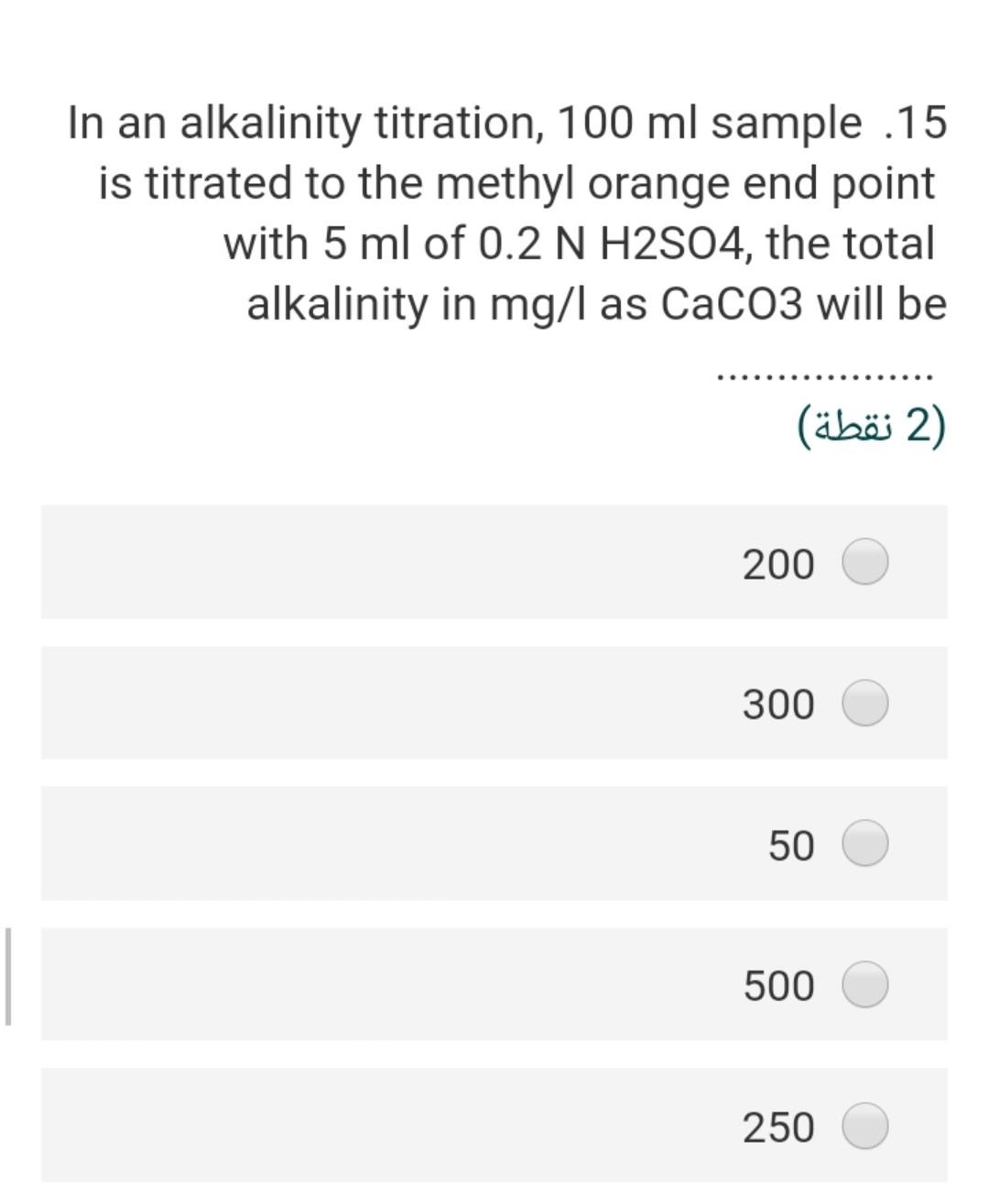

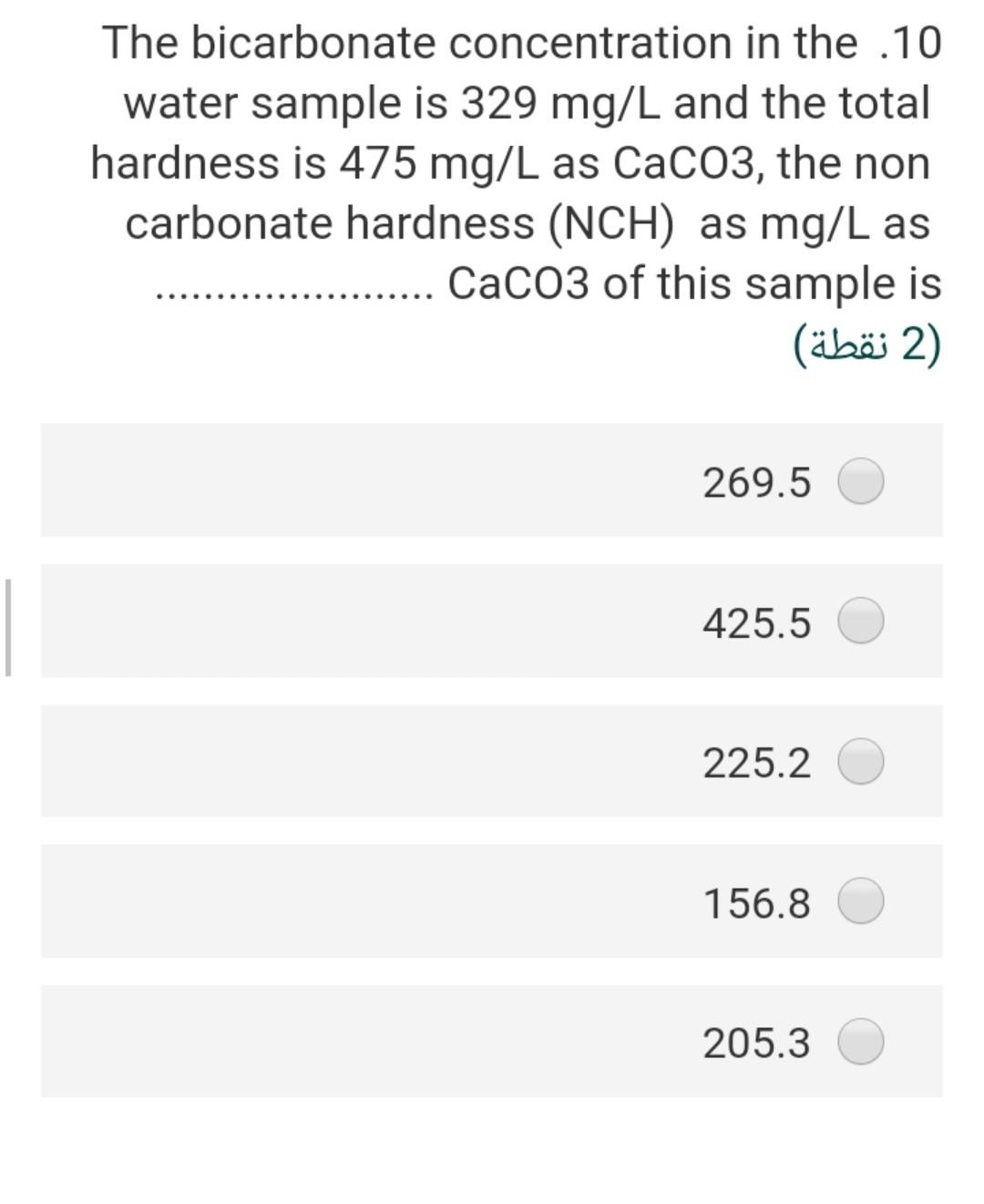
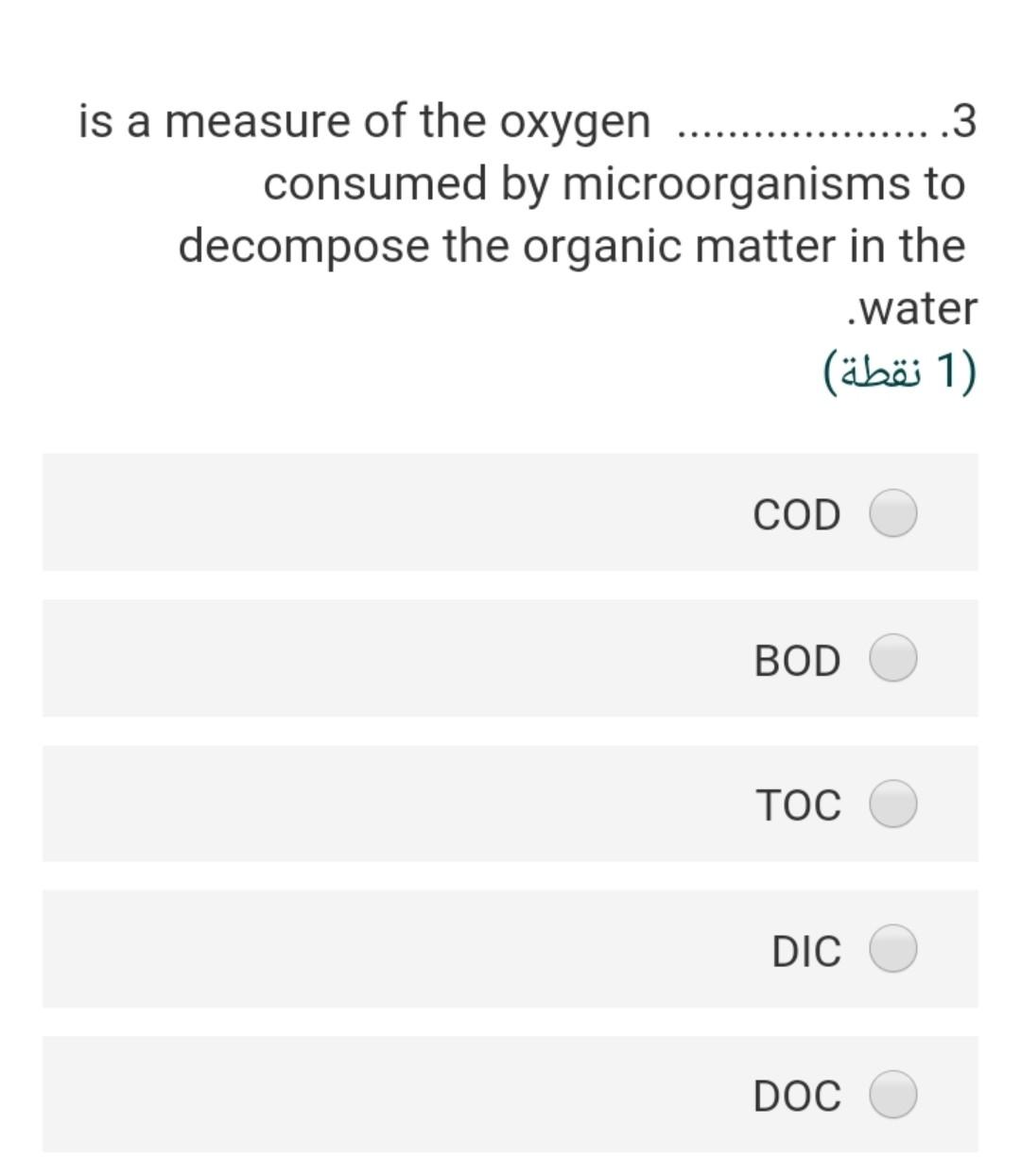
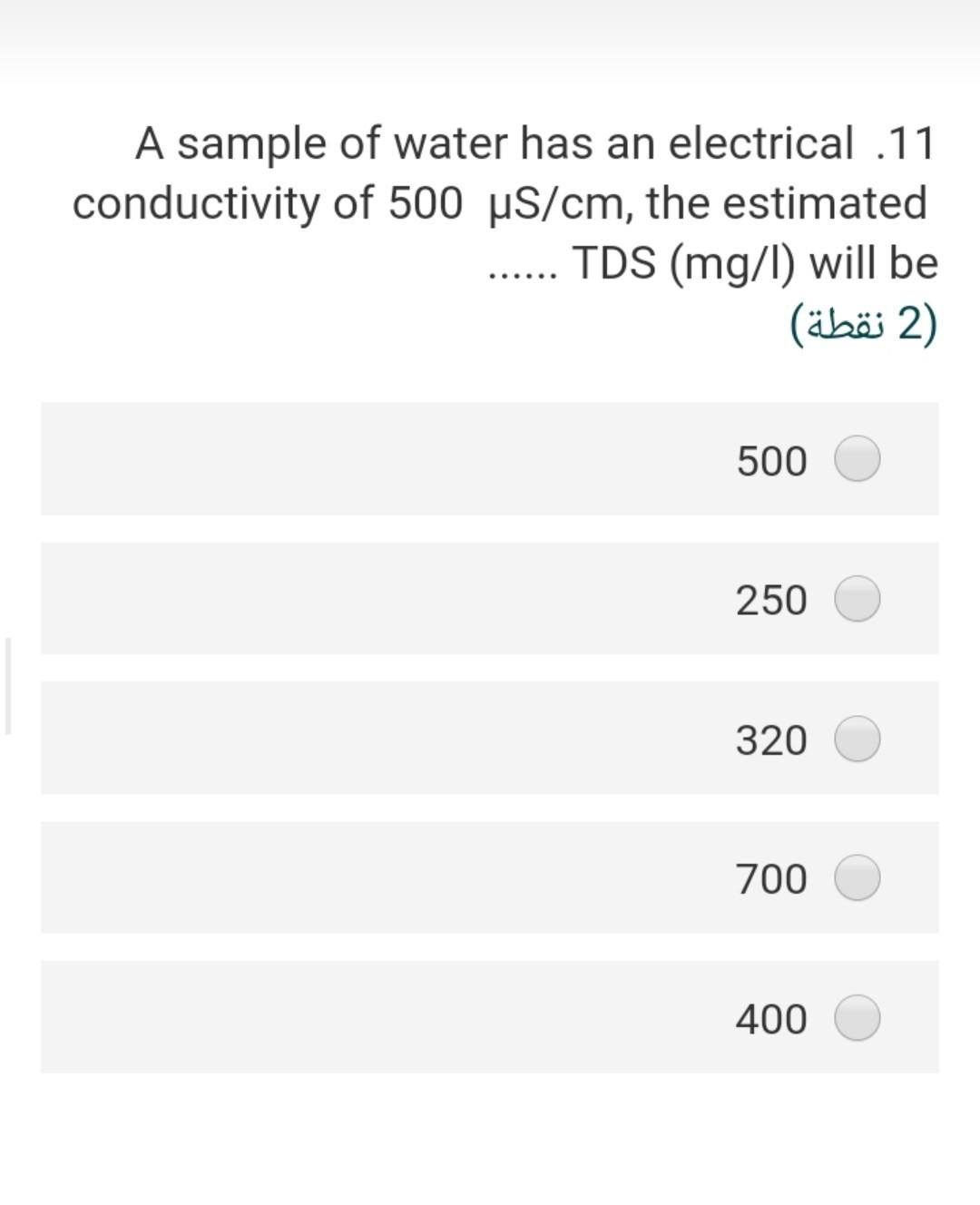

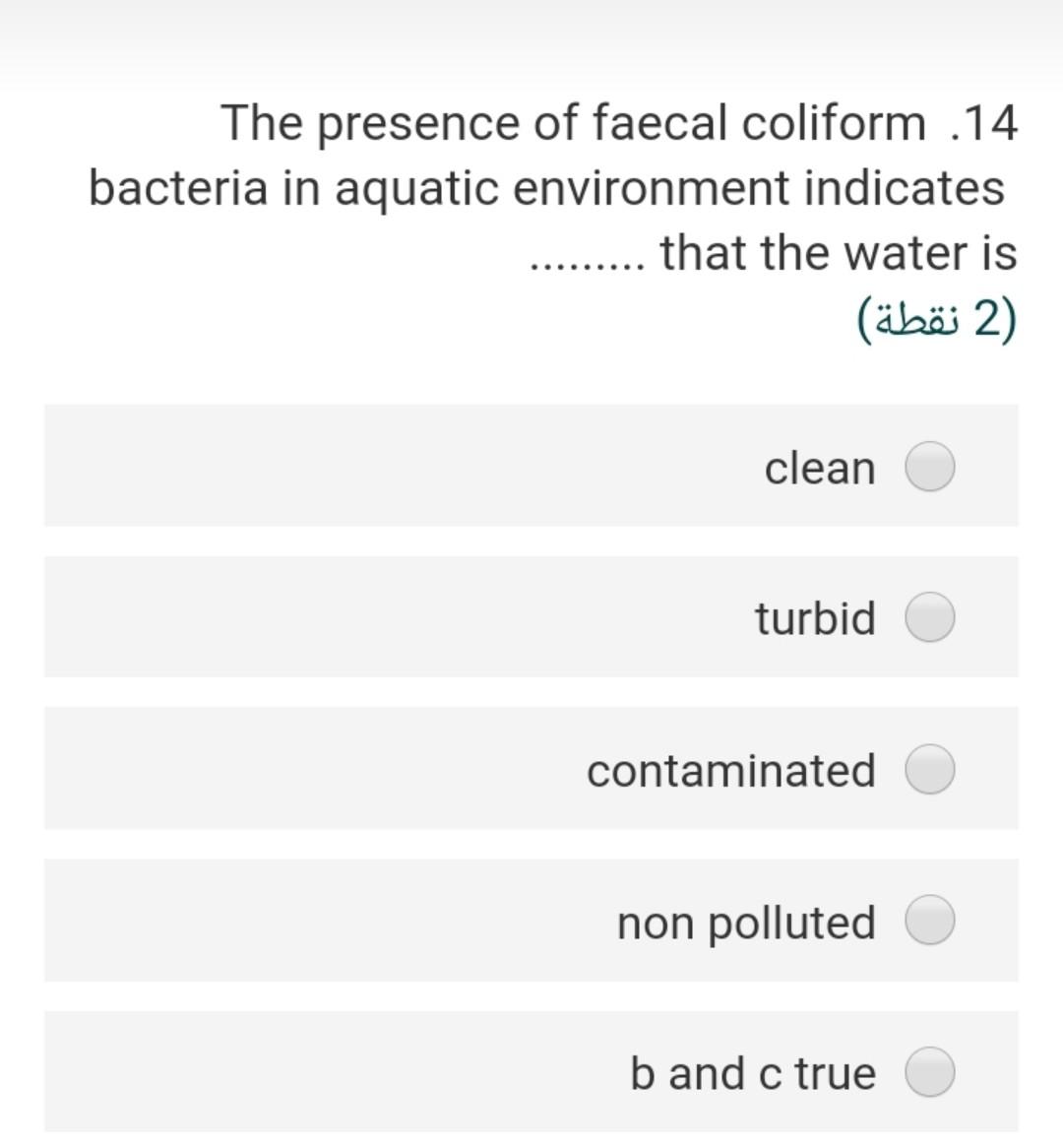
Given the following reaction .23 Fe(OH)3 + e- + 3H+ Fe+2 + 3 H20 -166.5 A -18.85 -56.68 the Pe of this reaction (2) 2.40 3.92 22.4- 5.56- 3.55 In an alkalinity titration, 100 ml sample .15 is titrated to the methyl orange end point with 5 ml of 0.2 N H2S04, the total alkalinity in mg/l as CaCo3 will be (2 ) 200 300 50 500 250 The water sample has the [Ca2+] = 45 .20 mg/L and [Mg2+] = 18 mg/L. The total hardness of this sample as mg/L as ... CaCo3 will be (2) 62 27 186.5 212.5 415.6 The bicarbonate concentration in the 10 water sample is 329 mg/L and the total hardness is 475 mg/L as CaCo3, the non carbonate hardness (NCH) as mg/L as CaCo3 of this sample is 2) (2 ) 269.5 425.5 225.2 156.8 205.3 is a measure of the oxygen .3 consumed by microorganisms to decompose the organic matter in the water (1 (1 ) COD BOD TOC DIC DOC A sample of water has an electrical .11 conductivity of 500 uS/cm, the estimated TDS (mg/l) will be 2 (2 ) 500 250 320 700 400 The type of chemical bond in the SiO2 .24 compound (Quartz mineral) knowing that the electronegativity of silica is 1.8 and .the oxygen is 3.5 (2 ) ionic, 21 % covalent 79% ionic, 96 % covalent 4% ionic, 49 % covalent 51% ionic, 11% covalent 89% ionic, 89 % covalent 11% The presence of faecal coliform .14 bacteria in aquatic environment indicates that the water is (2) clean turbid contaminated non polluted b and c true
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


