Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the reactions (1) and (2) below, determine the following. (1) H2(g)+Cl2(g)2HCl(g)rH=184.62kJmol1 (2) 2H2(g)+O2(g)2H2O(g)rH=483.64kJmol1 (3) 4HCl(g)+O2(g)2Cl2(g)+2H2O(g) (a) rH and rU for reaction (3) rH kJmol1
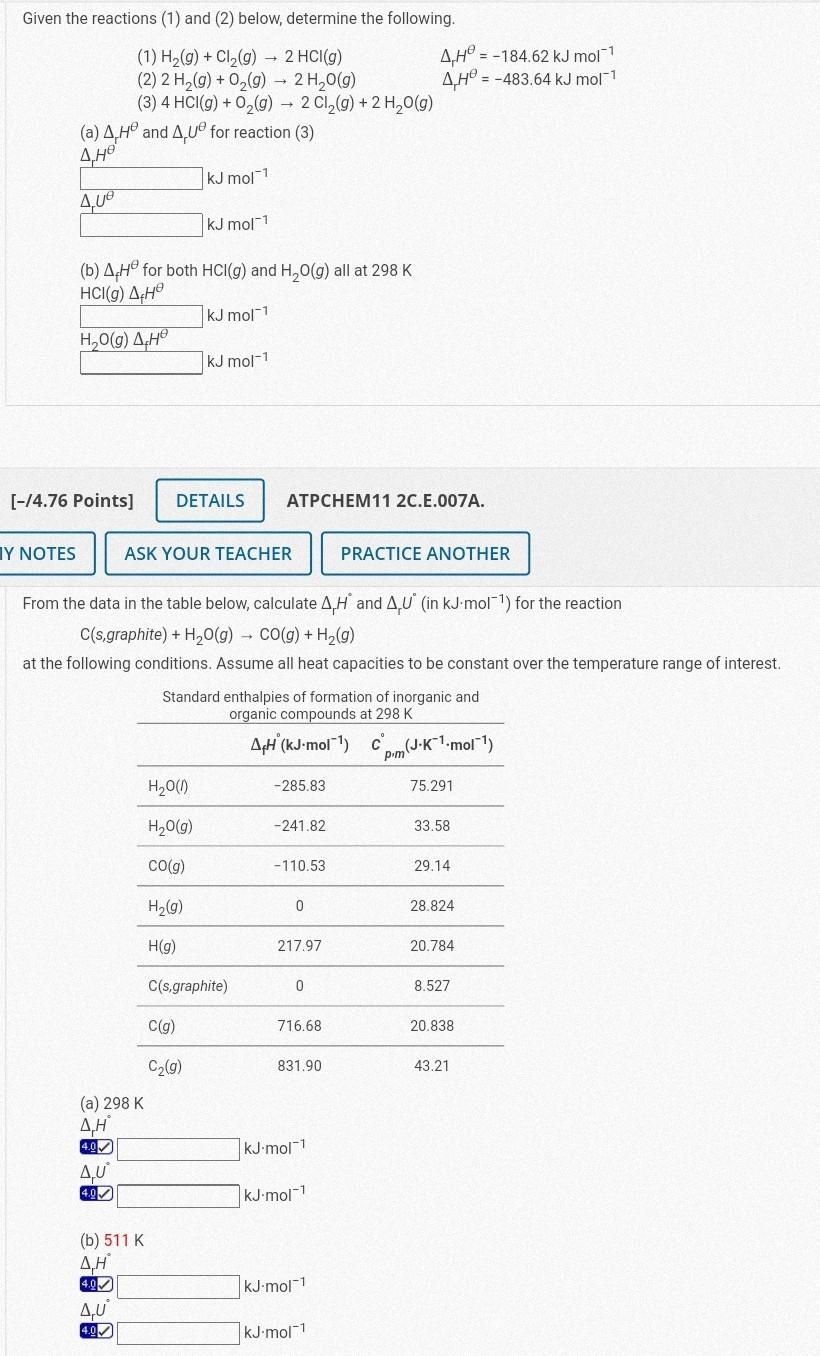
Given the reactions (1) and (2) below, determine the following. (1) H2(g)+Cl2(g)2HCl(g)rH=184.62kJmol1 (2) 2H2(g)+O2(g)2H2O(g)rH=483.64kJmol1 (3) 4HCl(g)+O2(g)2Cl2(g)+2H2O(g) (a) rH and rU for reaction (3) rH kJmol1 rU kJmol1 (b) fH for both HCl(g) and H2O(g) all at 298K HCl(g)fH H2O(a)cHkJmol1kJmol1 [14.76 Points ] ATPCHEM11 2C.E.007A. From the data in the table below, calculate rH and rU (in kJmol1 ) for the reaction C(s,graphite)+H2O(g)CO(g)+H2(g) at the following conditions. Assume all heat capacities to be constant over the temperature range of interest
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


