Answered step by step
Verified Expert Solution
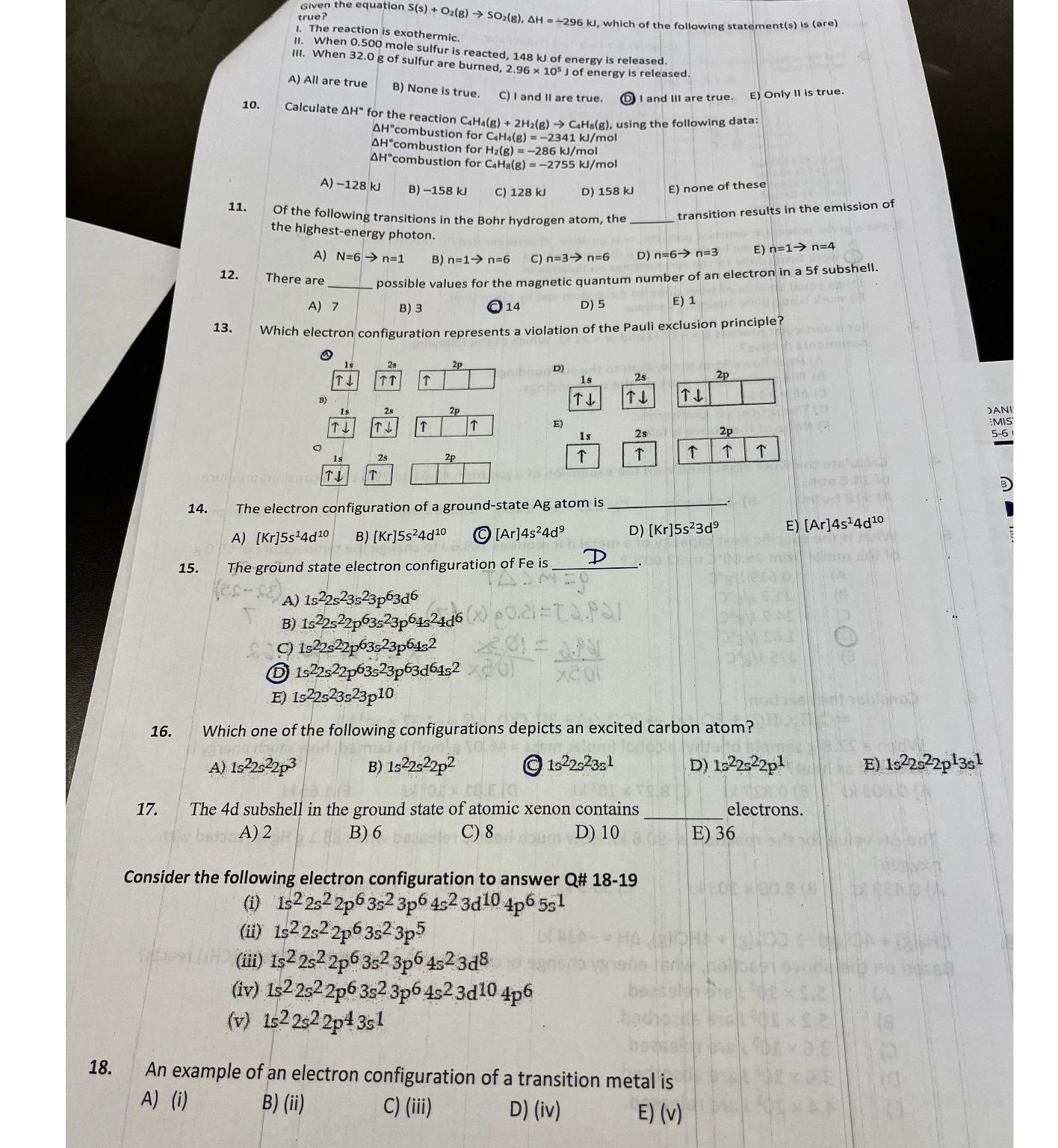
Question
1 Approved Answer
Given true? The reaction is When 0 . 5 0 0 mole sulfur is reacted, 1 4 8 k J of energy is released. III.
Given
true?
The reaction is
When mole sulfur is reacted, of energy is released.
III. When of sulfur are burned, of energy is released.
A All are true
B None is true.
C I and II are true.
I and III are true.
E Only II is true.
Calculate for the reaction using the following data: combustion for
combustion for
combustion for
A
B
C
D
E none of these
Of the following transitions in the Bohr hydrogen atom, the transition results in the emission of the highestenergy photon.
A
B
C
D
E
There are possible values for the
A
B
D
E
Which electron configuration represents a violation of the Pauli exclusion principle?
D
c
The electron configuration of a groundstate atom is
A
B
C
D
E
The ground state electron configuration of is D
A
B
C
D
E
Which one of the following configurations depicts an excited carbon atom?
A
B
C
D
E
The subshell in the ground state of atomic xenon contains electrons.
A
B
C
D
E
Consider the following electron configuration to answer Q#
i
ii
iii
iv
v
An example of an electron configuration of a transition metal is
Ai
Bii
Ciii
Div
Ev
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


