Question
21. The atomic number (Z), electron configurations, and numbers of unpaired electrons for five ions are listed in the following table. Assume that all
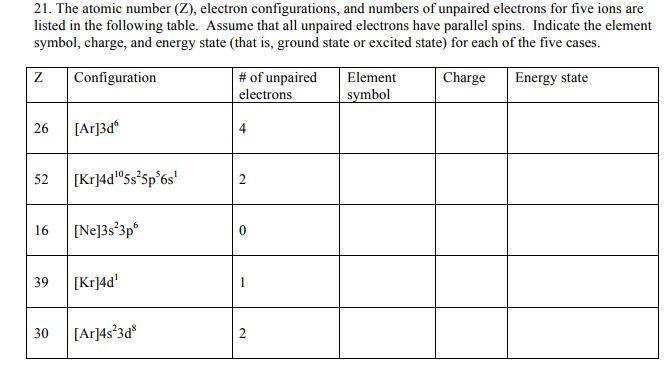
21. The atomic number (Z), electron configurations, and numbers of unpaired electrons for five ions are listed in the following table. Assume that all unpaired electrons have parallel spins. Indicate the element symbol, charge, and energy state (that is, ground state or excited state) for each of the five cases. Configuration Charge Energy state Z 26 52 16 39 30 [Ar]3d [Kr]4d05s5p6s [Ne]3s3p6 [Kr]4d [Ar]4s3d8 # of unpaired electrons 4 2 2 Element symbol
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Z Configuration Number of Unpaired Electrons Element Symbol Charge Energy State 26 Ar3d 6 4 Fe 2 G...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Marketing Research Essentials
Authors: Carl McDaniel Jr., Roger Gates
8th edition
1118249321, 978-1118475911, 1118475917, 978-1118249321
Students also viewed these Marketing questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



