Answered step by step
Verified Expert Solution
Question
1 Approved Answer
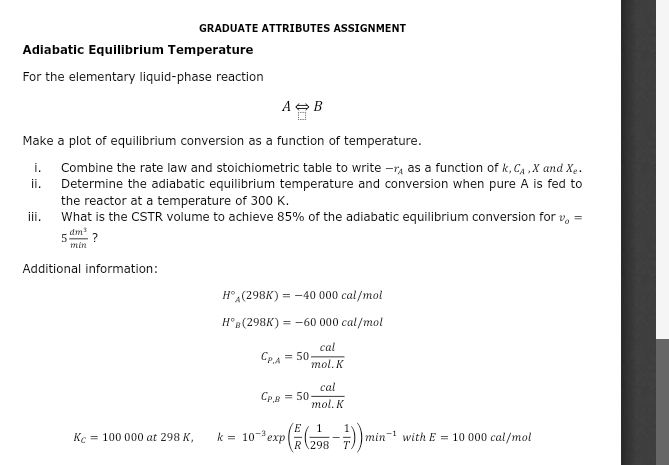
GRADUATE ATTRIBUTES ASSIGNMENT Adiabatic Equilibrium Temperature For the elementary liquid - phase reaction A > B Make a plot of equilibrium conversion as a function
GRADUATE ATTRIBUTES ASSIGNMENT
Adiabatic Equilibrium Temperature
For the elementary liquidphase reaction
Make a plot of equilibrium conversion as a function of temperature.
i Combine the rate law and stoichiometric table to write as a function of and
ii Determine the adiabatic equilibrium temperature and conversion when pure is fed to the reactor at a temperature of
iii. What is the CSTR volume to achieve of the adiabatic equilibrium conversion for
Additional information:
exp with
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


