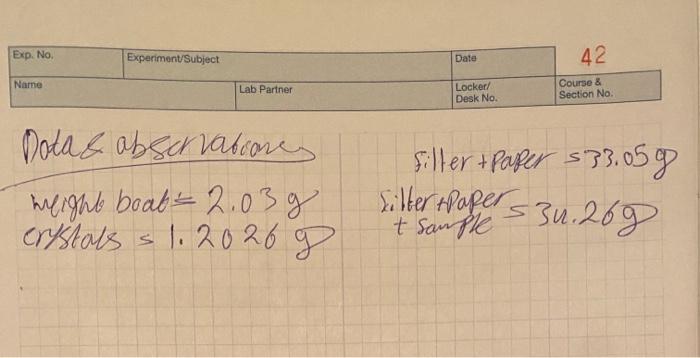
Gravimetric Analysis Of Sulfate: Analysis Of Alum EXPERIMENT A-245 POST-LAB QUESTIONS Show your work on your calculations. Carry the proper number of significant figures in your calculations. 1 Calculate the masses of the alum samples that you analyzed. 2 Calculate the mass of barium sulfate obtained from each sample. 3 Calculate the mass of sulfate ion present in each of the barium sulfate samples. 4 Calculate the experimental percent by mass of sulfate in each of the alum samples. 5 Calculate the average percent sulfate. 6 Calculate the theoretical percent by mass of sulfate in pure alum, KAI(SO) - 12HO. 7 (a) Calculate the percent difference (% error) in the average percent sulfate. (b) Is your experimental average percent sulfate high or low relative to the theoretical value? (c) How can you explain your percent difference in light of your lab work? (Give specific examples of what might have happened to cause your percentage to be high or low.) 8 Would your experimental percent sulfate be high or low if your alum sample had a soluble non-sulfate impurity, for example, water? To prove your answer, do the following calculation. Assume that included in the weight of a sample of alum are 0.10 g of water. The total weight of sample is 1.20 g. Calculate the percent sulfate in the sample. 9 Would your experimental percent sulfate be high or low if you spilled a little barium sulfate? Explain. 10 Would your experimental percent sulfate be high or low if the barium sulfate were incompletely washed? Explain. 11 Would your experimental percent sulfate be high or low if the barium sulfate were incompletely dried? Explain. Exp. No. Experiment/Subject Name Lab Partner Dota & abservationes weight boab 2.03g crystals 1.2026 g Date 42 Locker/ Desk No. Course & Section No. Filter + Paper $33.059 Filter +Paper 31.269 + Sample Gravimetric Analysis Of Sulfate: Analysis Of Alum EXPERIMENT A-245 POST-LAB QUESTIONS Show your work on your calculations. Carry the proper number of significant figures in your calculations. 1 Calculate the masses of the alum samples that you analyzed. 2 Calculate the mass of barium sulfate obtained from each sample. 3 Calculate the mass of sulfate ion present in each of the barium sulfate samples. 4 Calculate the experimental percent by mass of sulfate in each of the alum samples. 5 Calculate the average percent sulfate. 6 Calculate the theoretical percent by mass of sulfate in pure alum, KAI(SO) - 12HO. 7 (a) Calculate the percent difference (% error) in the average percent sulfate. (b) Is your experimental average percent sulfate high or low relative to the theoretical value? (c) How can you explain your percent difference in light of your lab work? (Give specific examples of what might have happened to cause your percentage to be high or low.) 8 Would your experimental percent sulfate be high or low if your alum sample had a soluble non-sulfate impurity, for example, water? To prove your answer, do the following calculation. Assume that included in the weight of a sample of alum are 0.10 g of water. The total weight of sample is 1.20 g. Calculate the percent sulfate in the sample. 9 Would your experimental percent sulfate be high or low if you spilled a little barium sulfate? Explain. 10 Would your experimental percent sulfate be high or low if the barium sulfate were incompletely washed? Explain. 11 Would your experimental percent sulfate be high or low if the barium sulfate were incompletely dried? Explain. Exp. No. Experiment/Subject Name Lab Partner Dota & abservationes weight boab 2.03g crystals 1.2026 g Date 42 Locker/ Desk No. Course & Section No. Filter + Paper $33.059 Filter +Paper 31.269 + Sample