Question
Iron (Fe) metal is corroded in an aerated dilute acid solution with a pH of 7. Since the corrosion potential (Ekor) of this corrosion
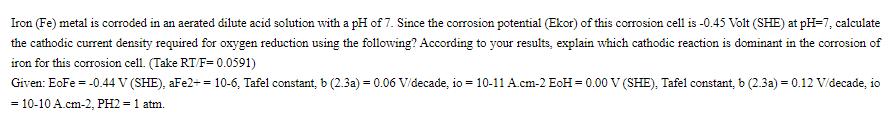
Iron (Fe) metal is corroded in an aerated dilute acid solution with a pH of 7. Since the corrosion potential (Ekor) of this corrosion cell is -0.45 Volt (SHE) at pH=7, calculate the cathodic current density required for oxygen reduction using the following? According to your results, explain which cathodic reaction is dominant in the corrosion of iron for this corrosion cell. (Take RT/F= 0.0591) Given: EoFe = -0.44 V (SHE), aFe2+ = 10-6, Tafel constant, b (2.3a) = 0.06 V/decade, io = 10-11 A.cm-2 EoH = 0.00 V (SHE). Tafel constant, b (2.3a) = 0.12 V/decade, io = 10-10 A.cm-2, PH2= 1 atm.
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Given Ekor 045 V SHE EoFe 044 V SHE aFe 106 bFe 23aFe 006 Vdecade ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
International Business
Authors: Simon Collinson, Rajneesh Narula, Alan M. Rugman
8th Edition
1292274158, 9781292274157
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



