Answered step by step
Verified Expert Solution
Question
1 Approved Answer
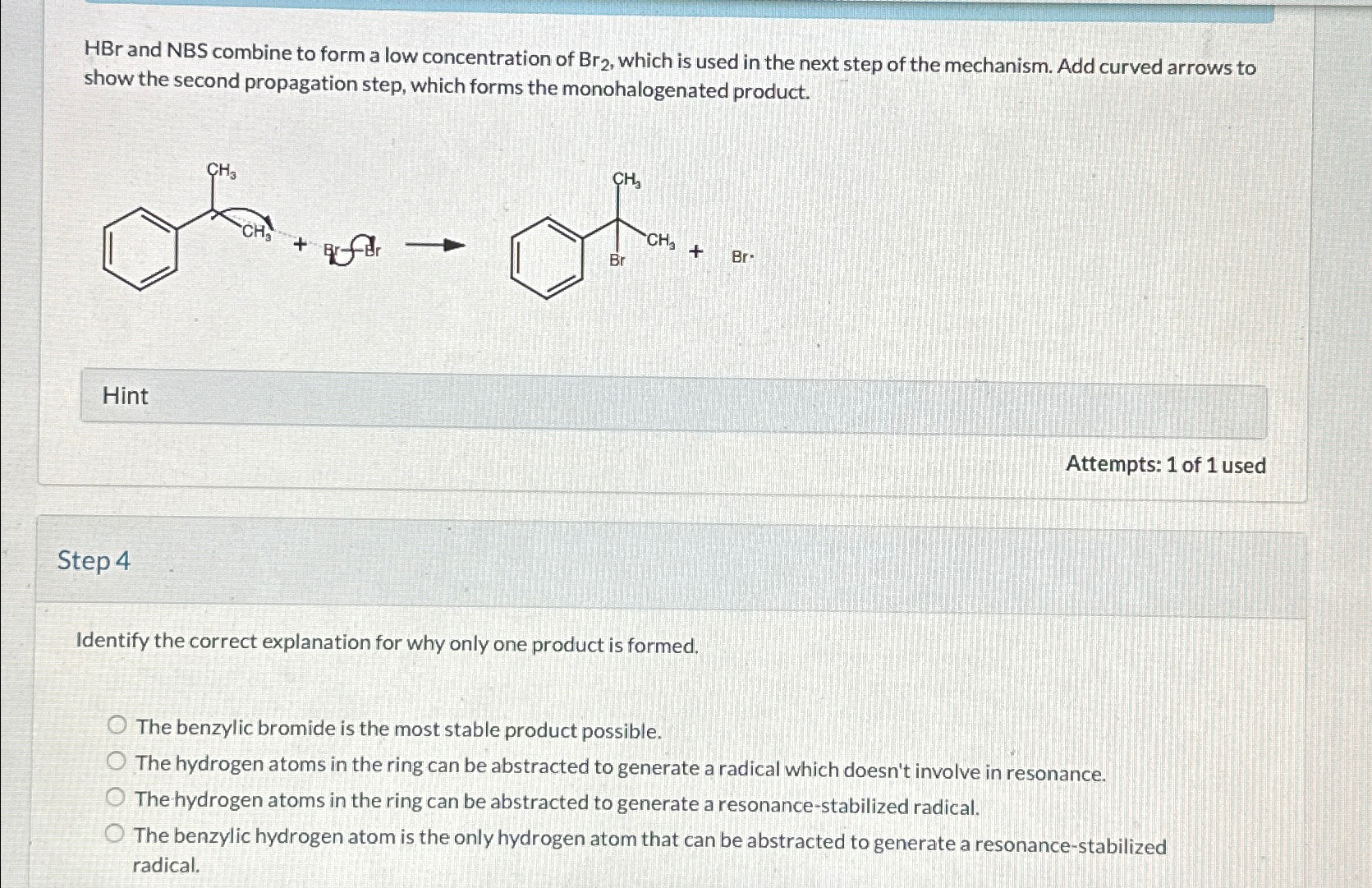
H B r and NBS combine to form a low concentration of B r 2 , which is used in the next step of the
and NBS combine to form a low concentration of which is used in the next step of the mechanism. Add curved arrows to show the second propagation step, which forms the monohalogenated product.
Hint
Attempts: of used
Step
Identify the correct explanation for why only one product is formed.
The benzylic bromide is the most stable product possible.
The hydrogen atoms in the ring can be abstracted to generate a radical which doesn't involve in resonance.
The hydrogen atoms in the ring can be abstracted to generate a resonancestabilized radical.
The benzylic hydrogen atom is the only hydrogen atom that can be abstracted to generate a resonancestabilized radical.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


