Answered step by step
Verified Expert Solution
Question
1 Approved Answer
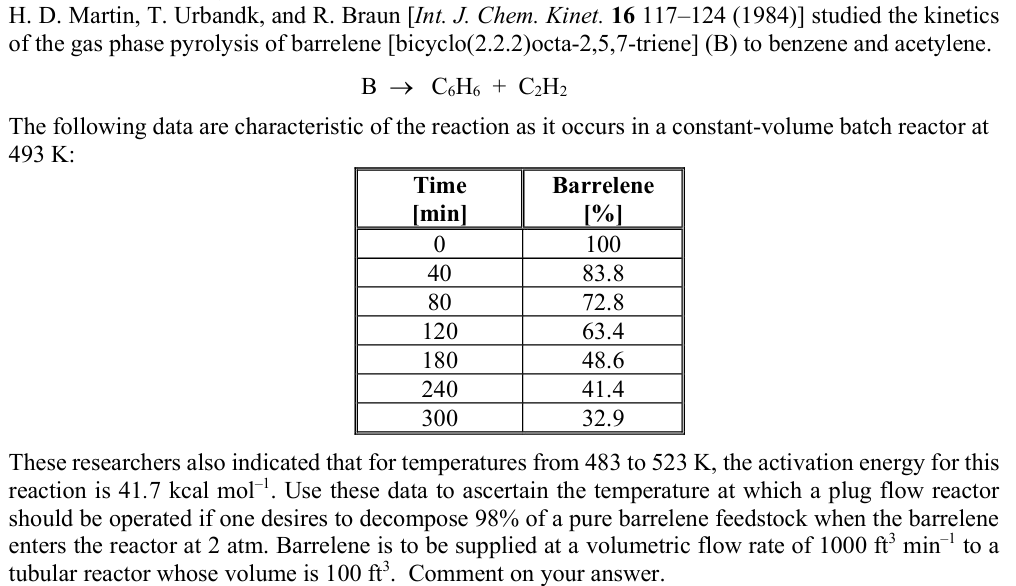
H . D . Martin, T . Urbandk, and R . Braun studied the kinetics of the gas phase pyrolysis of barrelene [ bicyclo (
H D Martin, T Urbandk, and R Braun studied the kinetics of the gas phase pyrolysis of barrelene bicyclooctatrieneB to benzene and acetylene.
The following data are characteristic of the reaction as it occurs in a constantvolume batch reactor at :
These researchers also indicated that for temperatures from to the activation energy for this reaction is Use these data to ascertain the temperature at which a plug flow reactor should be operated if one desires to decompose of a pure barrelene feedstock when the barrelene enters the reactor at atm. Barrelene is to be supplied at a volumetric flow rate of to a
tubular reactor whose volume is Comment on your answer.
What is the mean residence time of a fluid element in the reactor for operation at this temperature?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


