Question: H2S : bond length b1 = 1.34A, bond angle theta1 = 92.1, displaced charge q1=....., dipole moment p1= 1.10D H2O : bond length b1 =
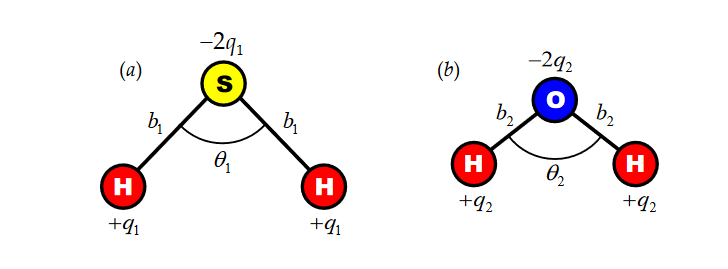
H2S : bond length b1 = 1.34A, bond angle theta1 = 92.1, displaced charge q1=....., dipole moment p1= 1.10D
H2O : bond length b1 = 0.96A, bond angle theta2 = ....., displaced charge q1=0.33e, dipole moment p1= 1.85D
a) Give a formula for the magnitude of the dipole moment of this kind of molecules in terms of q, b, and theta. Explain.
b) For each molecule, clearly draw the direction of its dipole moment in Fig, and what's q1 and theta2?
c) Which atom is more electronegative, S (sulfur) or O (oxygen)?
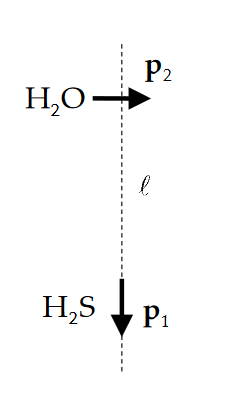
d) Calculate the electric field that the hydrogen sulfide molecule sets up at the location of the water molecule (magnitude, direction, and unit) where l = 60.0 nm
f) Assuming this field is uniform (it isnt...!), calculate the torque on the water molecule (magnitude, direction, and unit).
g) If we held the hydrogen sulfide molecule fixed in space, how would the water molecule tend to reorient itself? Why?
-291 -292 (a) (b) S b by. b, bi , , +92 +92 +91 +91 P2 HO l HS P1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


