Question
he isomerization of butane from a trans to the gauche conformational state has an equilibrium constant of approximately K = [gauche]/[trans] = 0.6 at 300
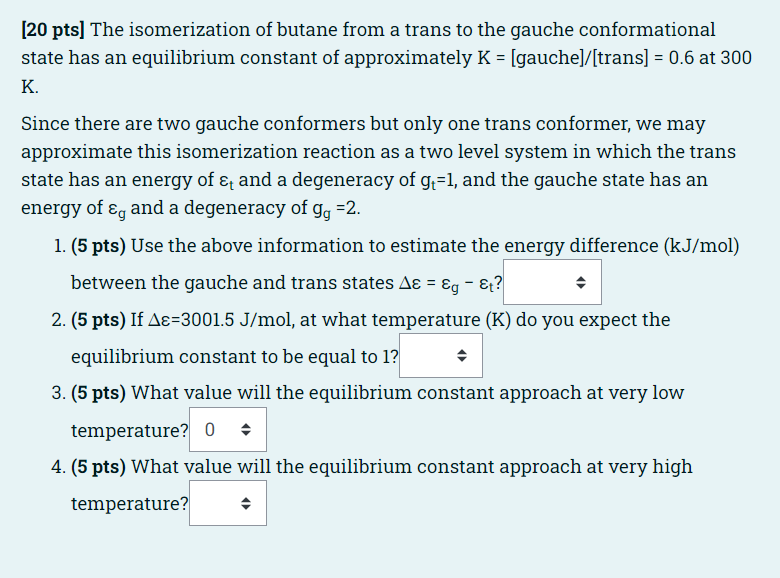
he isomerization of butane from a trans to the gauche conformational state has an equilibrium constant of approximately K = [gauche]/[trans] = 0.6 at 300 K.
Since there are two gauche conformers but only one trans conformer, we may approximate this isomerization reaction as a two level system in which the trans state has an energy of t and a degeneracy of gt=1, and the gauche state has an energy of g and a degeneracy of gg =2.
(5 pts) Use the above information to estimate the energy difference (kJ/mol) between the gauche and trans states = g t?Answer 3.00230021273-1273
(5 pts) If =3001.5 J/mol, at what temperature (K) do you expect the equilibrium constant to be equal to 1?Answer 450522550600
(5 pts) What value will the equilibrium constant approach at very low temperature?Answer 00.512
(5 pts) What value will the equilibrium constant approach at very high temperature?Answer 00.512
[20 pts] The isomerization of butane from a trans to the gauche conformational state has an equilibrium constant of approximately K= [gauche] /[ trans] =0.6 at 300 K. Since there are two gauche conformers but only one trans conformer, we may approximate this isomerization reaction as a two level system in which the trans state has an energy of t and a degeneracy of gt=1, and the gauche state has an energy of g and a degeneracy of gg=2. 1. (5 pts) Use the above information to estimate the energy difference (kJ/mol) between the gauche and trans states =gt ? 2. (5 pts) If =3001.5J/mol, at what temperature (K) do you expect the equilibrium constant to be equal to 1? 3. (5 pts) What value will the equilibrium constant approach at very low temperature? 4. (5 pts) What value will the equilibrium constant approach at very high temperatureStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


