Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. Calculate calories of heat from the burned candle absorbed bythe water. B. Calculate calories of heat produced per gram of candle waxburned. C. Calculate
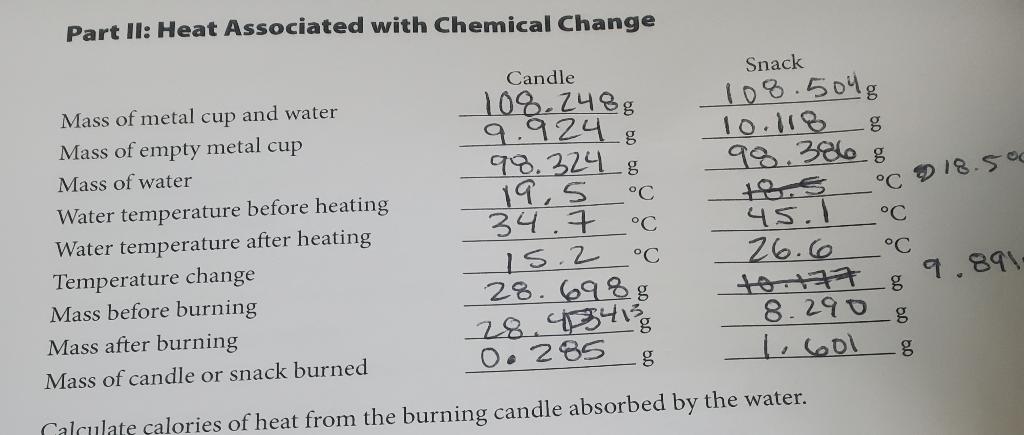
A. Calculate calories of heat from the burned candle absorbed bythe water.
B. Calculate calories of heat produced per gram of candle waxburned.
C. Calculate calories of heat from the burning snack absorbed bythe water.
D. Calculate the calories of heat produced per gram of snackburned.
E. Which is the better fuel- gram for gram, candle wax or thesnack food?
Part II: Heat Associated with Chemical Change Mass of metal cup and water Mass of empty metal cup Mass of water Water temperature before heating Water temperature after heating Candle 108.2488 9.9248 98.3248 C 19,5 34.7 C C 15.2 28.6988 28.434130 0.285 Snack 108.5048 10.118 g 98.3868 18.5 45.1 26.6 10-177 8.290 8 g g 1.601 Temperature change Mass before burning Mass after burning Mass of candle or snack burned Calculate calories of heat from the burning candle absorbed by the water. C 18.500 C C 9.891-
Step by Step Solution
There are 3 Steps involved in it
Step: 1
A Given mass of water 98324 grams Initial temperature of water 195 degr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


