Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Heat of combustion of benzoic acid Benzoic acid (g) :0.4961 g by using the data and the example, based on that data value of BA
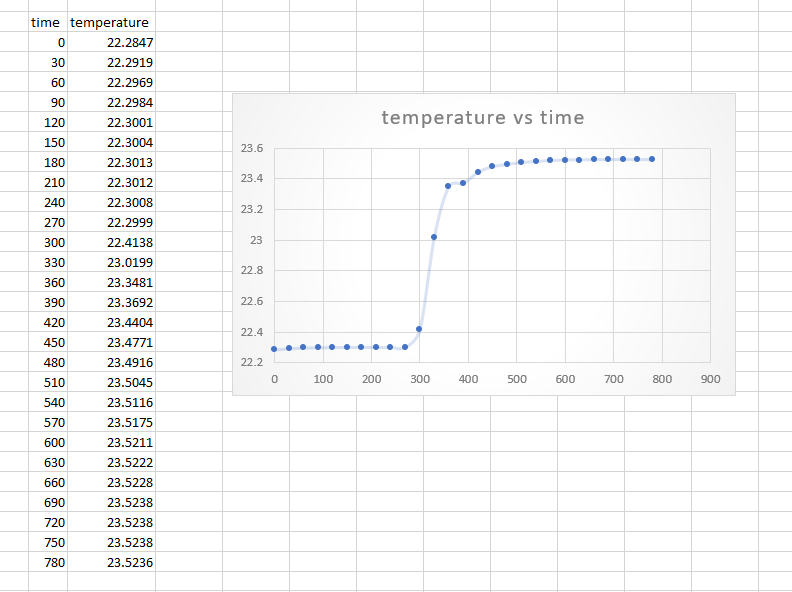
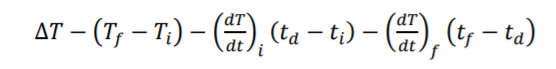
Heat of combustion of benzoic acid Benzoic acid (g) :0.4961 g by using the data and the example, based on that data value of BA what is the initial and final drift rates. (dT/dt)i and (dT/dt)f.? and what is the td ? *for the formula change in temperature is equal not minus
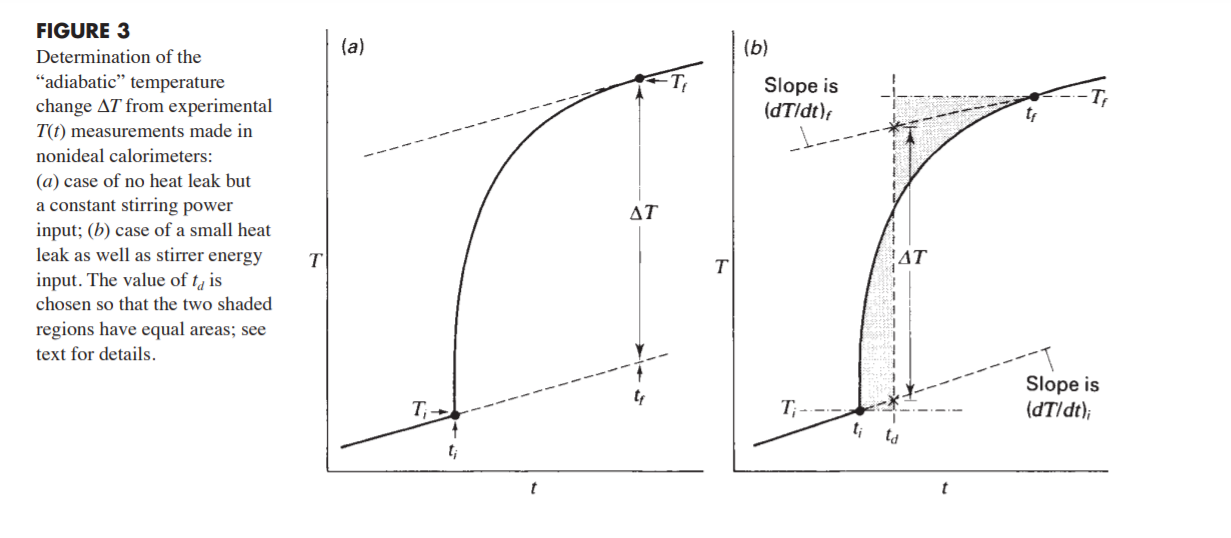
time temperature 0 22.2847 30 22.2919 60 22.2969 90 120 temperature vs time 150 180 23.6 . 23.4 210 240 23.2 270 23 22.2984 22.3001 22.3004 22.3013 22.3012 22.3008 22.2999 22.4138 23.0199 23.3481 23.3692 23.4404 23.4771 23.4916 23.5045 23.5116 23.5175 23.5211 22.8 300 330 360 390 420 450 480 510 540 22.6 22.4 . 22.2 0 100 200 300 400 500 600 700 800 900 570 600 630 23.5222 23.5228 660 690 23.5238 23.5238 720 750 23.5238 780 23.5236 (a) (6) Slope is (dT/dt) In- FIGURE 3 Determination of the "adiabatic temperature change AT from experimental T(t) measurements made in nonideal calorimeters: (a) case of no heat leak but a constant stirring power input; (b) case of a small heat leak as well as stirrer energy input. The value of t, is chosen so that the two shaded regions have equal areas; see text for details. AT T T AT Slope is (dT/dt); T, T; t; td t; t - (, - ) - (), (ta - t) - (H), (; ta)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


