Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hello, can I please get help with this problem set questions??? Thankyou Problem 2: Unknown molecule B Mass spectroscopy: This compound has a mass spectrum

Hello, can I please get help with this problem set questions???
Thankyou
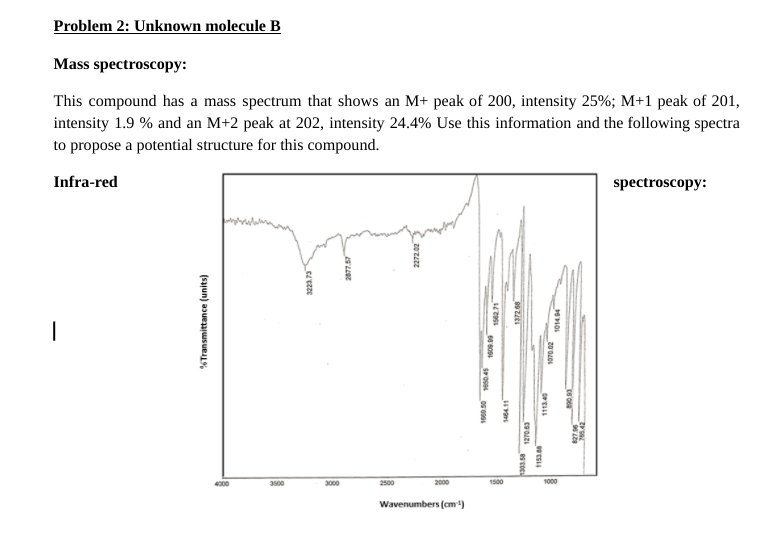
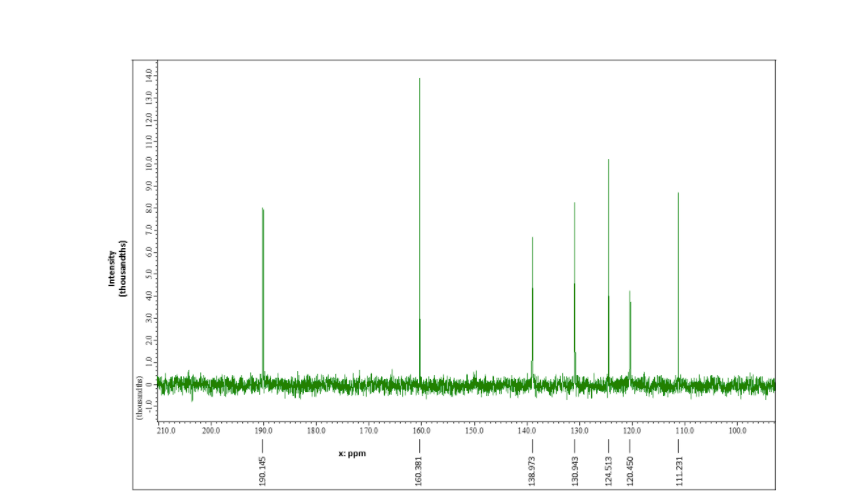
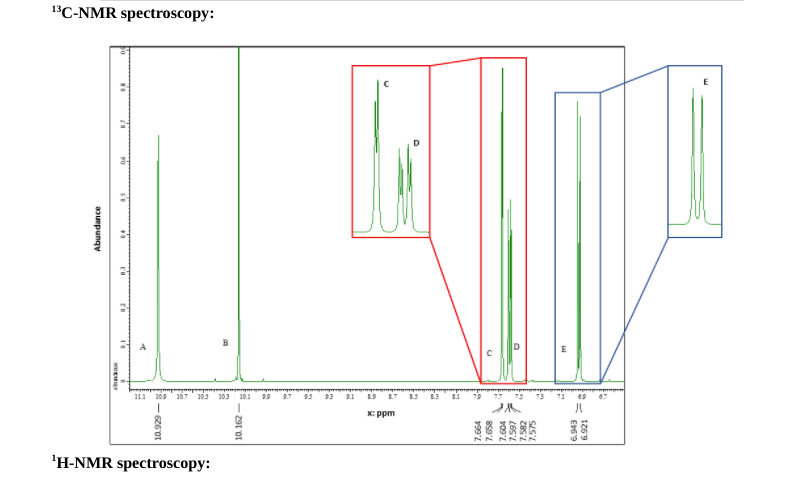
Problem 2: Unknown molecule B Mass spectroscopy: This compound has a mass spectrum that shows an M+ peak of 200, intensity 25%; M+1 peak of 201, intensity 1.9 % and an M+2 peak at 202, intensity 24.4% Use this information and the following spectra to propose a potential structure for this compound. Infra-red spectroscopy: 2677.57 2272.02 1223.73 %Transmittance (units) 1562.71 137268 1014.04 1009.09 1970.02 STO 1454.11 1113.40 1270.63 1153.00 9329 76542 BS DE 4000 3500 3000 2500 2000 1500 1000 Wavenumbers (cm) Intensity (thousandths) (thousands) DO 10 20 30 40 SD 60 70 80 90 100 110 120 130 140 2100 190.145 x ppm 160.381 1500 138.973 130.943 130.0 124.513 120.450 1200 111231 1100 100.0 13 C-NMR spectroscopy: D Abundance 3 B D 11 303 307 308 303 301 99 9.795 9.1 10 1.1 1.53 81 79 19 75 73 71 69 6.1 5-60601 x: ppm 10.162 7.664 7.658 BE 6.943 1769 H-NMR spectroscopy: 'H-NMR spectroscopy: Answer the following questions: 1. Draw your proposed structure. Use the letters on the proton NMR spectra to label the specific protons on your structure such that they match. (2+1 pts) 2. Explain why you proposed this structure, with reference to how each type of data supports your conclusion: a. MS (3 pts): b. IR (3 pts): C. C NMR (3 pts): d. 'H NMR (5 pts): 3. If unknown molecule B performs an aldol condensation reaction with acetaldehyde in presence of K.CO3, what will be the final product? (3 pts) H unknown B ? K2CO3 SolventStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


