Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hello guys could uou help me ? based on the report form, could you help with the laboratory report please ? need help asap. the
hello guys could uou help me ? based on the report form, could you help with the laboratory report please ? need help asap. the last 2 pages are just a sample example. we use different compund as you see on my 2first pages. 


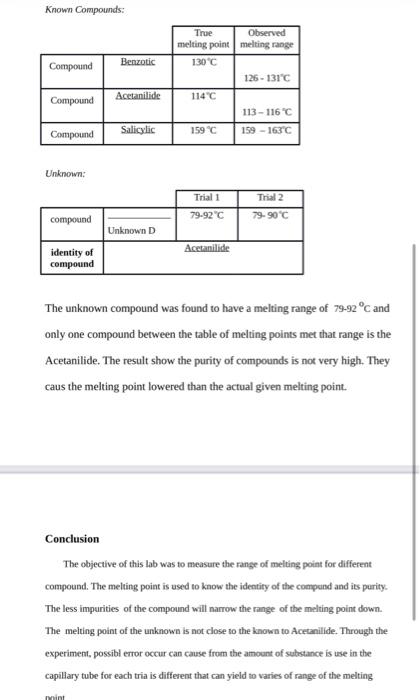
bruar D2 I. Melting Points of Pure compounds M.P.C Name of compound (Experimental) (a) Lrta 1310-13C M.P.,"C (Literature) 189.5-133C (b) Cinnamic Acid 135.0-13C 1825-13350 II. Melting Point of an Impure Substance Melting Point of a Mixture of (a) and (b) 132-137 C III. Identification of an Unknown Solid from its Melting Point Unknown Number = 1 ial 1: M.P.of unknown when mixed with 69-72 C M.P. of PURE unknown = 68-71_C in Phenylbenzoate Benzhydrol BrPhenyl (name of compound) al 2: M.P. of unknown when mixed with 64-68 cc (name of compound) 13: M.P. of unknown when mixed with C (name of compound) My unknown compound is Phenylben Coste (name of compound) Format of Laboratory Report Your tabertory report should be divided into the following section 1. Introduction A brief statement of the purpose of the experiment. This is also > pood place to show relevant structures and chemical equations IL. Experimental Procedure A brief outline of the experimental procedure. Be particular about reporting the amounts of materials used and any modifications made to the original procedure (avoid simply copying the original procedure) TII. Results and Discussion This section is the most important. Include observations such as appearance of the reaction, color of product, etc. If the experiment was a preparative one, you should also report your percent yield: Show all of your calculations! Gruphs should be done on graph paper. Note: Our lab manual contains a "Data Report Sheet" for each experiment. You may record your results here and include this sheet at this stage of your report. The discussion part comes from you! Were your results what you expected? If not, can you suggest reasons why not? If you look a melting point of a compound you synthesized, what is the true or "literature melting point? How well does your melting point compare? What does your melting point indicate about the purity of your compound? Assume that your reader is not entirely familiar with the experiment, so you need to explain clearly. IV. Conclusions Your overall evaluation of your results. This is a good place to mention any modifications to the procedure which you feel might improve the outcome of the experiment V. Answers to Exercises These questions appear at the end of each experiment in the laboratory manual or handout. Usually you will be given selected "prelaboratory questions and regular questions from the lab manual to answer. You should write your report in ink, or type it, using one side of the paper only. If you write your report by hand (which is perfectly OK as long as it is neat and legible), use lined paper (not tom out of a spiral notebook!). Always use complete sentences. Try your best to avoid spelling and grammatical errors. Write your report in impersonal form. The words "T" or "we" should not appear in your report. The following examples show some incorrect phrases and how they can be revised to avoid the personal form: INCORRECT: I added 10 g of NaCito... CORRECT: Ten grams of NaCl were added to... INCORRECT: You told me that ... CORRECT: The instructor indicated that ... INCORRECT: We determined that ... DETERMINATION OF MELTING POINTS Introduction The melting point of a compound is the temperature at which it changes from a solid to a liquid. The physical property used to check the purity of the compound the identify compounds. The substance melt reach a certain temperature range the the solid change to liquid phases. Above that temperature range, the substance present only as a liquid, and below it the solid phase is only present. The temperature range is a measure use to e determine the purity of the substance. The impure substance of compounds have lower temperature range of melting. The compound can only obtain a melting range of a 2-3C accuracy. This is sufficient for most uses of the melting point. This experiment is to determine the melting points of compounds and to use these to identify unknowns. Experimental procedure Used the finely divided powder to best conducted the melting points for different compounds. Small amount of different organic compounds were ground using a mortar and pestle. Then filled in a capillary tube to a height of about 2 mm. The tubes were placed into the melting point apparatu chamber and observed through the magnifying glass. The instructor indicated that the temperature should be set 10C below the exact temperature for the known compounds. The temperature range recorded at the first sight when solid substance melt to liquid to the temperature at which the substance were completely change liquid. The melting range is the melting point fo the compounds. Repeated the processes for all the known and the unknown compounds. Known Compounds: Compound Benzotic True Observed melting point melting range 130C 126 - 131C 114C 113 - 116C 159 C 159 - 163C Acetanilide Compound Compound Salicylic Unknown: Trial 1 Trial 2 compound 79-92"C 79-90C Unknown D Acetanilide identity of compound The unknown compound was found to have a melting range of 79-92 C and only one compound between the table of melting points met that range is the Acetanilide. The result show the purity of compounds is not very high. They caus the melting point lowered than the actual given melting point. Conclusion The objective of this lab was to measure the range of melting point for different compound. The melting point is used to know the identity of the compund and its purity. The less impurities of the compound will narrow the range of the melting point down. The melting point of the unknown is not close to the known to Acetanilide. Through the experiment, possibl error occur can cause from the amount of substance is use in the capillary tube for each tria is different that can yield to varies of range of the melting Mint 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


