Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hello! Im currently stuck on this lab and need help fillijg out data sheet 1 and 2 please!!! I will upvote!! Objective Croate a standard
Hello! Im currently stuck on this lab and need help fillijg out data sheet 1 and 2 please!!! I will upvote!! 
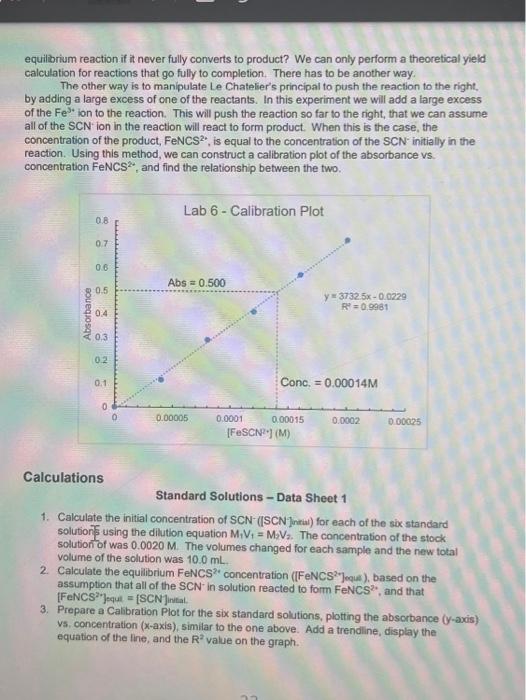


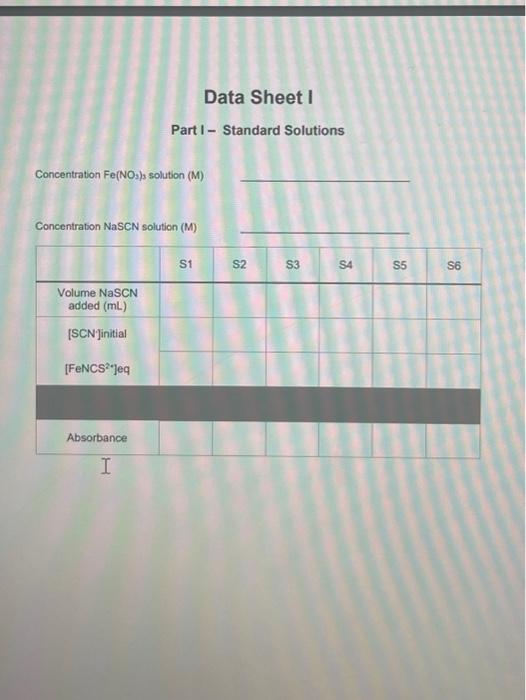
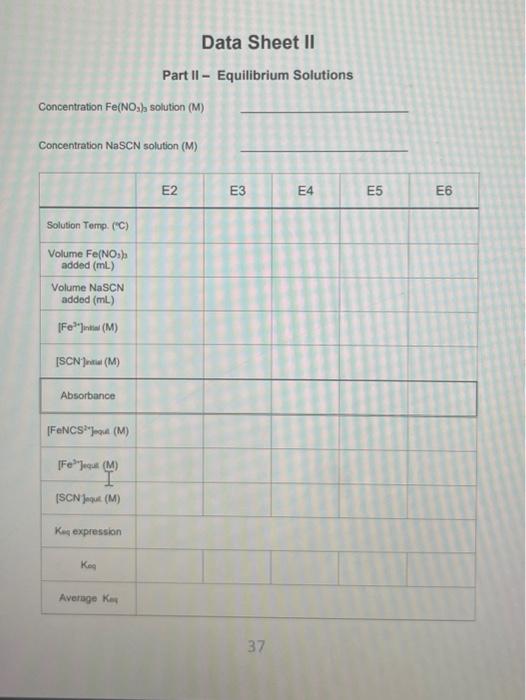
Objective Croate a standard ourve for solutions with known concentrations of the product, ron thiocyanato, and use the standard curve to determine the equilibrium concentrations of the product in solutions where the concentrations are unknown. Then determine the equilicium constant for the reaction. Introduction As you leamed in the previous lab, equilibrium roactions, have both the forward and reverse reaction are occurring simultaneously. Eventually, these reactions reach a dynamic equilbrium where the rates of both the forward and reverse reaction are the same. At this point the concentrations of the reactants and the products remains constant. The ratio of the products to reactants in the final reaction mbdure depends on the Keq, the equilibium constant For example, the equiliorium reaction between N2 and H2 to form NH3 is seen below, along with its Keq equation. The Keq equation puts the concentration of the productis) raised to the power of their coefficient divided by the concentration of the reactant(s) raised to the power of their coefficients: N2(g)+3H2(g)2NH3(g)Keq=[N2][H2]3[NH3]2 These reactions never fully proceed to completion and it is extremely difficut to delemine the amount of product(s) that will be produced based on the amount of teactants you start with. By finding the value of the equilibrium constant the ratio of the products to reactants can be found and one can determine whether the reaction miduro will be mostly products, mostly reactants, or more of an even mbcture of both. In today's experiment, iron(ii) ion is reacted with the thiocyanate ion (SCN) to produce isothibcyanatolon(iii) ion (FeNCS"). This reaction works well for this experiment because the product is an orange-red color, while Fe2 " is palo yollow and SCN is coloriess. Aher the reoctants are mixed and equabrium has been roached, the absorbanoes of the reactions can be measured and according to Beer's Law the absorbance and the concentration of a solution are directy proportional Fe3+(aq)+SCN(aq)FeNCS2+(aq)Keq=[Fe3+sCN2][FeNCS2+] Solutions with known concentrations of product will be produced and thei absorbance measured. A calbration plot is then produced showing the exact relationship between the absorbance and concentration for the product. Then the callbration plot is used lo determine the concertuations of the product in solutions where the concentrations are unknown. There as one dilemena left to solve though. How can the concentration of the product be known for an equilbrium reaction if it never fully converts to product? We can only perform a theoretical yield calculation for reactions that go fully to completion. There has to be another way. The other way is to manipulate Le Chatelier's principal to push the reaction to the right, by adding a large excess of one of the reactants. In this experiment we will add a large excess of the Fe3+ ion to the reaction. This will push the reaction so far to the right, that we can assume all of the SCN ion in the reaction will react to form product. When this is the case, the concentration of the product, FeNCS 2, is equal to the concentration of the SCNinitially in the reaction. Using this method, we can construct a calibration plot of the absorbance vs. concentration FeNCS2, and find the relationship between the two. Calculations Standard Solutions - Data Sheet 1 1. Calculate the initial concentration of SCN - (ISCN Jintial) for each of the six standard solution 1 using the dilution equation M1V1=M2V2. The concentration of the stock solution of was 0.0020M. The volumes changed for each sample and the new total volume of the solution was 10.0mL. 2. Calculate the equilibrium FeNCS 2 concentration ([FeNCS 2v Jeque ), based on the assumption that all of the SCNin solution reacted to form FeNCS2, and that [ FeNCS 2r] equil =[SCN] intal. 3. Prepare a Calibration Plot for the six standard solutions, plotting the absorbance ( y-axis) vs, concentration ( x-axis), similar to the one above. Add a trendline, display the equation of the line, and the R2 value on the graph. using the dilution formula again. The concentration of Fe3+ is 0.0020M and 5mL was added to each sample. The concentration of SCNis still 0.0020M and the volumes vary by sample. 5. Using your Calibration Plot, determine the equilibrium concentrations of the FeNCS 2, (FeNCS 2 ]equi) by rearranging the equation of the line to solve for x. So y=mx+b becomes x=myb, where y=absorbance of the sample, m= slope, and b=y-intercept. The m and b values are given to you in the equation of the line. taking what was there initilly and subtracting the amount that reacted (product formed) [Fe2+]intial[FeNCS2] oqui =[Fe3] equi [SCN] initiat [FeNCS2] poul =[SCN] oqa The difference is what remains at equilbrium. 7. Write the Keq expression on the data sheet and find the Keq for the reaction by plugging in the equilibrium concentrations of all of the substances Procedure Part I - Standard Solutions 39. Prepare 6 standard solutions by adding the correct amount of solutions, according to Table 1 below, to 6 large test tubes. Mx them thoroughly with a glass stirring rod. Rinse the glass stirring rod in between mbing the solutions. The total volume of each solution should be 10.0mL. 40. The solutions are located in the back of the lab in burets. They can be added accurately in small volumes using this method. The volumes of NaSCN are small so be careful when adding! Make sure you are adding the correct solution. there are two different Fe(NO3)3 solutions, for this part you need the 0.200MFe(NO3)2. 41. Set the wavelength of your spectrophotometer to 447nm and make sure that the machine is set to record the absorbance of your solutions. 12. Obtain one exvette from your taboratory instructor. Rinse the cuwatte with a smalt amount of SI and pour into a beaker that you are not using for anything else. This will be your 'Waste Beaker'. Rinse again and then fill the cuvette (about \% of the way) with S1. 43. Put the cuvette in the spectrophotometer and press the 0A/100\%T button. Wat 10-20 seconds for the machine to read 0.00 . This will blank the machine, telling at that since 81 contains no product it will have 0 absorbance. 44. Pour the cuvette into your waste beaker and rinse it twice with 52 . Then fill it with $2 (about % of the way) place the cuvette in the machine, and record the absorbance on Data Sheet 1 . 45. Repeat Step 6 with the remaining samples untif you have absorbances for all 6 solutions: Part II - Equilibrium Solutions 46. Prepare 6 solutions of unknown concentrations by adding the correct amount of solutions, according to Table 2 below, to 6 large test tubes. Mix them thoroughly with a glass stiring rod. Rinse the glass stirring rod in between mixing the solutions. The total volume of each solution should be 10.0mL. 47. The volumes of NaSCN are small so be careful when adding! Make sure you are adding the correct solution. This time you are adding the 0.0020MFe(NO2). 48. Rinse the cuvette with a small amount of E1 and pour into your waste beaker. Rinse again, pour it into the waste beaker, then fill (about % of the way) with E1. 49. Place E1 in the spectropholometer and press the OAbs/100\%T butlon fo blank the machine. 50. Pour the curvette into your waste beaker and rinse it twice with E2. Then fill is with E2 (about of of the way) place the cuvetie in the machine, and record the absorbance on Data Stheet 2 (tr's fowards the middle) 51. Repeat Step 12 with the remaining samples until you have absorbances for all 6 solutions. 52. Obtain a thermometer and measure the temperature of one of your solutions. WASTE DISP0SRL: All of the waste must he dlisposed of in an: Aqueous Wh5TE Centainer Part I - Standard Solutions Data Sheet II Part II - Equilibrium Solutions Concentration Fe(NO3) solution (M) Concentration NaSCN solution (M) 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


