hello, please help ASAP! please answer all INCORRECT questions with an explanations for the answer selection. please and thank you.

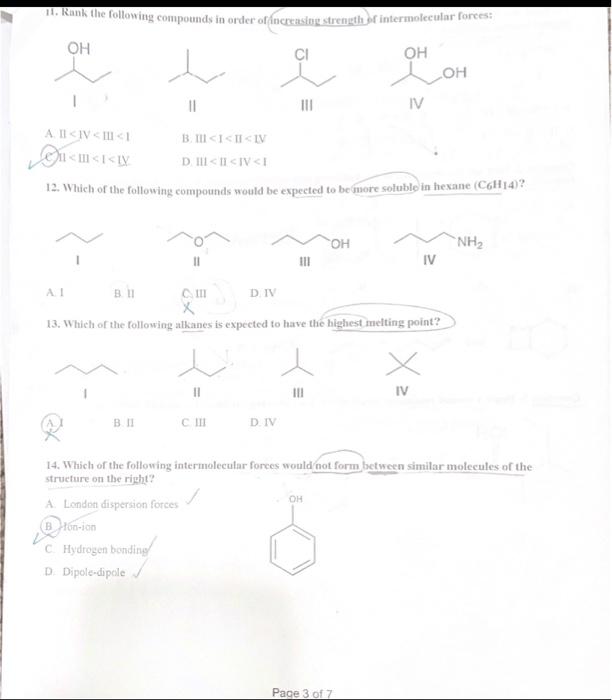
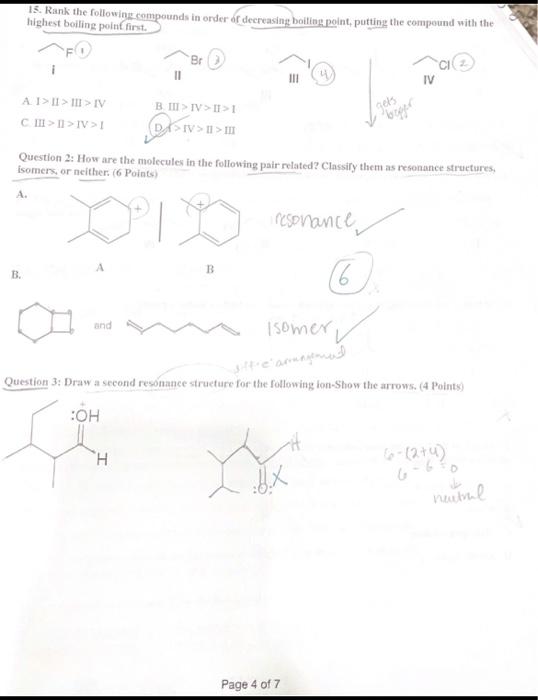
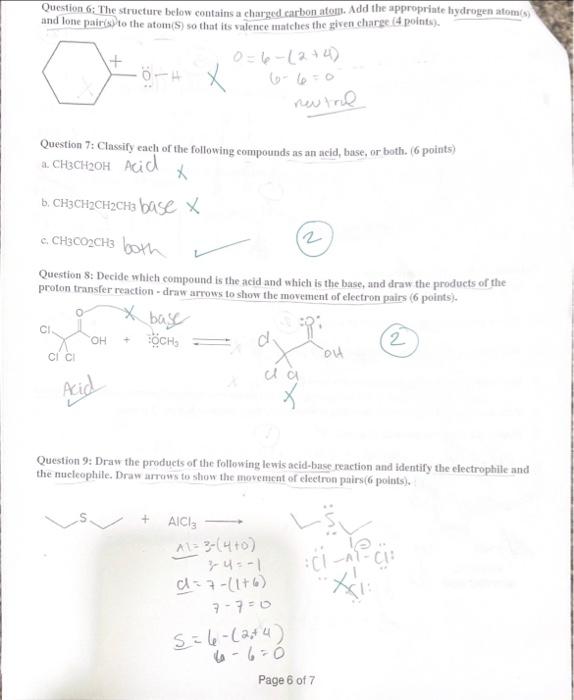
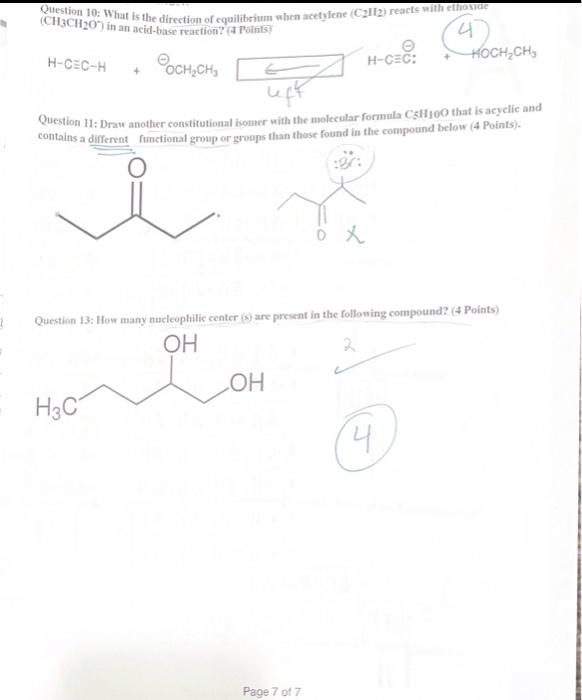
Question 1: Choose the correct Answer ( 30 points) 1. Why do heteroatoms confer reactivity on a particular molecule? (A. Because they have lone pairs and create electron-rich sites on carbon. B. Because they have lone pairs, and create electron-deficient sites on carbon. C. Because they are electronegative and act as electrophiles. D. Because they are electropositive and act as nucleophiles. 3. Which of the following correctly matches the molecules to the names of the functional group? I. CH3OH Carboxylic acid II CH3CO2CH3 Ester III CH3COCH3 Ketone IV. H2CO Alcohol A. 1 and II B.AII and IV C. II and III D: II and IV 4. Consider the molecule atenolol (a blocker used to treat hypertension). Which of the following lists the correct functional goups present in atenolol? D) - Primary alcohol, amide, primary amine, aromatic, ether B. Secondary alcohro1, amidee, secondary amine, aromatic, ether C. Secondary atcohol, amide, primary amine, aromatic, ether atenolol (used to treat high blood pressure) D. Secondary alcohol, amide, secondary amine, aromatic, ester Page 1 of 7 5. Which of the following would you expect to have ionic bonds? a. CO (b.) BrNi3 d. KCl 6. How many clectrons are around Sulphur in sulphuric acid (H2SO4) ? (2. 6 b. 8 c. 10 d. 12 7. Which of the following compounds are non-polar? CO2INH3IIH2OIIIBCl3IV single best answer: A Lower pKa values result from the lower acidity of the CH bond. B. Higher pKa values result from better resonance stabilization of the conjugate base C. Lower pKa values result from better resonance stabilization of the conjugate base. (1.) Higher \( \mathrm{p} \mathrm{K}_{\perp} \) values result from better stabilization of the conjugate base by electronegative elements 9. Which of the following statements about the solubility of organic compnounds in H2O is true? 1. The non-polar part of a molecule that is not attracted to water is said to be bydrophillic. (3.) The non-polar part of a molecule that is not attracted to water is said to be hydrophobic C) The polar part of a molecule that can hydrogen bond to water is said to be hydrophiobic D For an organic compound with one functional group that contains an O or N atom, the compound is water soluble only if it has 35 carbens. 11. Rank the following compounds in order of fncreasing strength of intermolecular forces: I II III IV A. II II > III > IV B. III > IV > II > I C. III > II > IV > I D S > IV > II > III Question 2: How are the molecules in the following pair related? Classiry them as resonance struetures, isomers, or neither, ( 6 Points) A. resonance B. A B and lsomer Question 3: Draw a second resonance strueture for the following fon-Show the arrows. (4 Points) 6(2+4)=0 nuatinl Question 4: For the following Compound A) draw Lewis structure considering that the compound is 3 alcoliol, 1 amine, and-2 halide ( 6 points). B) Calculate the formal charge on the Nitrogen atoms (s) points) +1 C) Predict the geometry around the uxygen atom of the alcohol group. (3 points) LinCc((?) Question 6: The structure below contains a charged carbon atoli. Add the appropriate hydrogen atom(s) and lone pair(s) to the atom(S) so that its valence matches the giren chares (4 points). 0=6(2+4) 66=0 nevire Question 7: Classify each of the following compounds as an acid, base, or both. (6 points) a. CH3CH2OH AciC x b. CH3CH2CH2CH3 baSe x c. CH3CO2CH3 both Question 8: Decide which compound is the acid and which is the base, and draw the products of the proton transfer reaction - draw arrows to show the movement of eleetron pairs ( 6 points). 2 Question 9: Draw the products of the following lewis acid-base reaction and identify the electrophille and the nucleophile. Draw arrows to show the movement of eleetron pairs ( 6 points), Question 10: What is the direction of equilitrium when acetylene (C2II2) reacts with ethovade (CH3CH2O) in an acid-base reaction? (a Potfis) HCCH+OCH2CH3 Question 11: Draw another constitutional isomer with the molecular formula C5H10O that is acyclic and contains a different ftunctional group or groups than those found in the compound below ( 4 Points). Question 13: How many nucleophilic center (s) are present in the folloning compound? ( 4 Points)