Question
Hello please help with partial fraction expansion of Laplace transform. Consider an isothermal CSTR whereby a single irreversible first-order reaction occurs in the reactor where
Hello please help with partial fraction expansion of Laplace transform.
Consider an isothermal CSTR whereby a single irreversible first-order reaction occurs in the reactor where A --> B where the arrow indicates a reaction of r=kCA.The unsteady-state model balance is ?(VdCA?)/dt=F(?v.)(CA?0?CA?)?VkCA??
CA - conc. of reactant A in the reactor (initially 1.0 mol / L)
CA0 - conc. of reactant A in the feed to the reactor (1.0 mol / L)
FV - volumetric flow rate (100 L/min)
k - rate constant (0.1 min)
V - volume of liquid in the reactor (1000 L)
Find ?CA?? (s) using Laplace transform and partial fraction to equal to
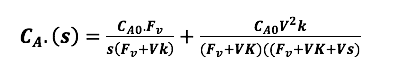
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


