Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hello there, Please help! 1. Determine the percent yield 2. label/summarize the results and analysis of the IR/NMR. (summarize your results and analysis of the
Hello there,
Please help!
1. Determine the percent yield
2. label/summarize the results and analysis of the IR/NMR. (summarize your results and analysis of the IR/NMR. )
Here are the results I got for IR/NMR
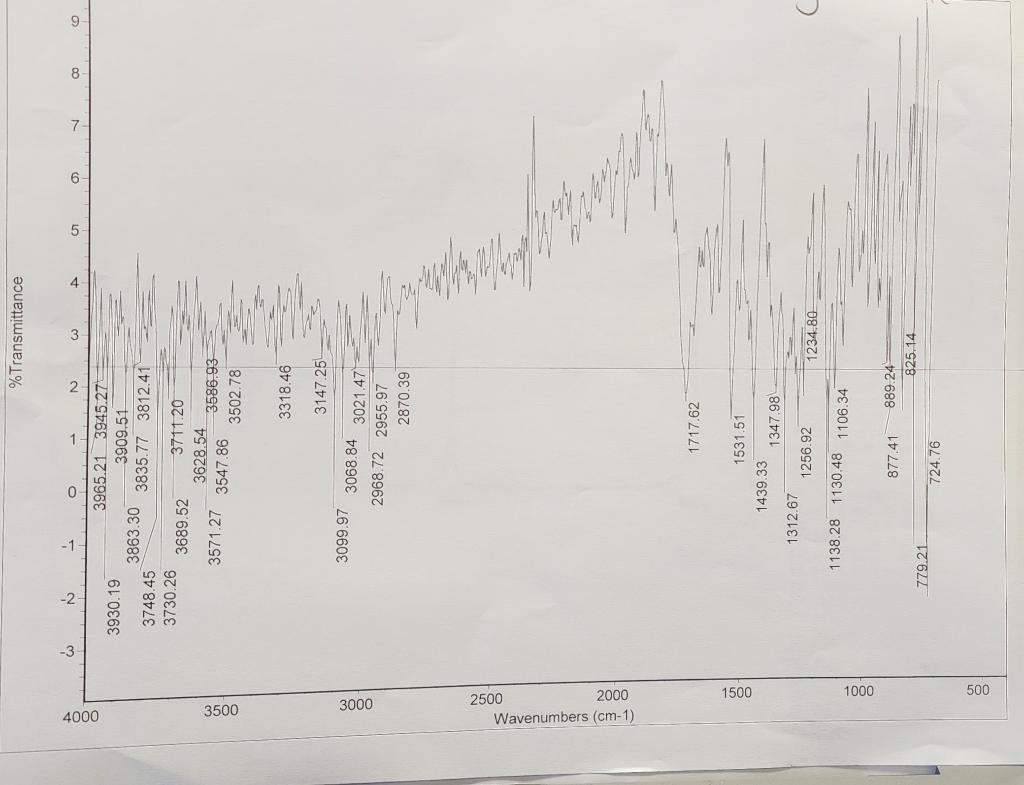
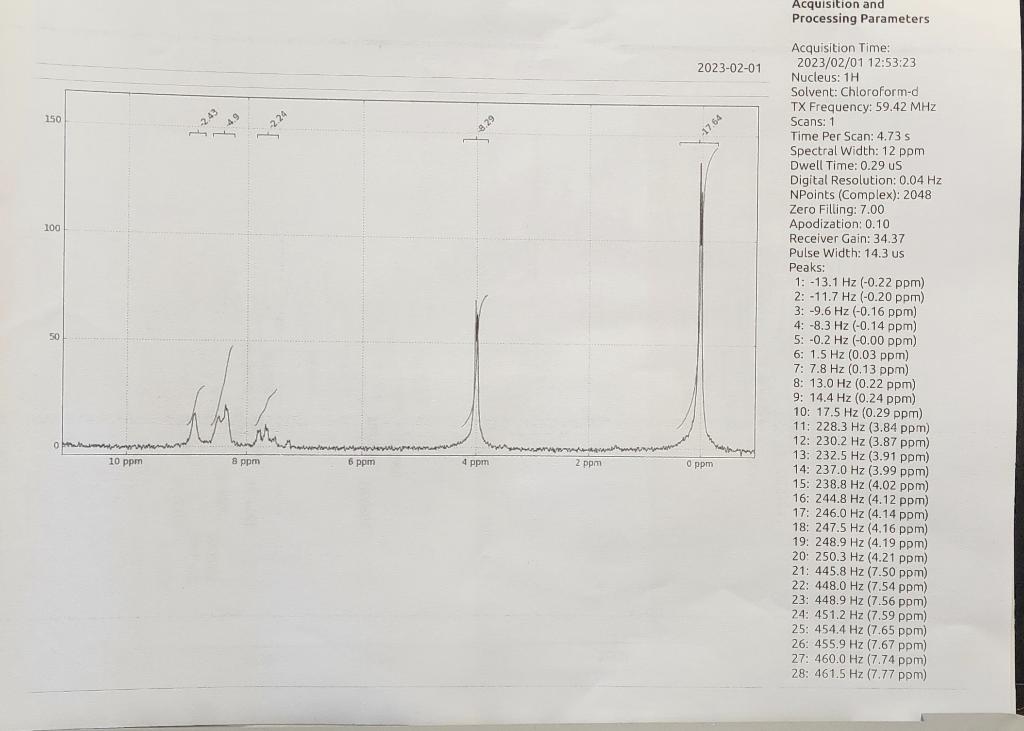
And here are the results I got they are highlighted. 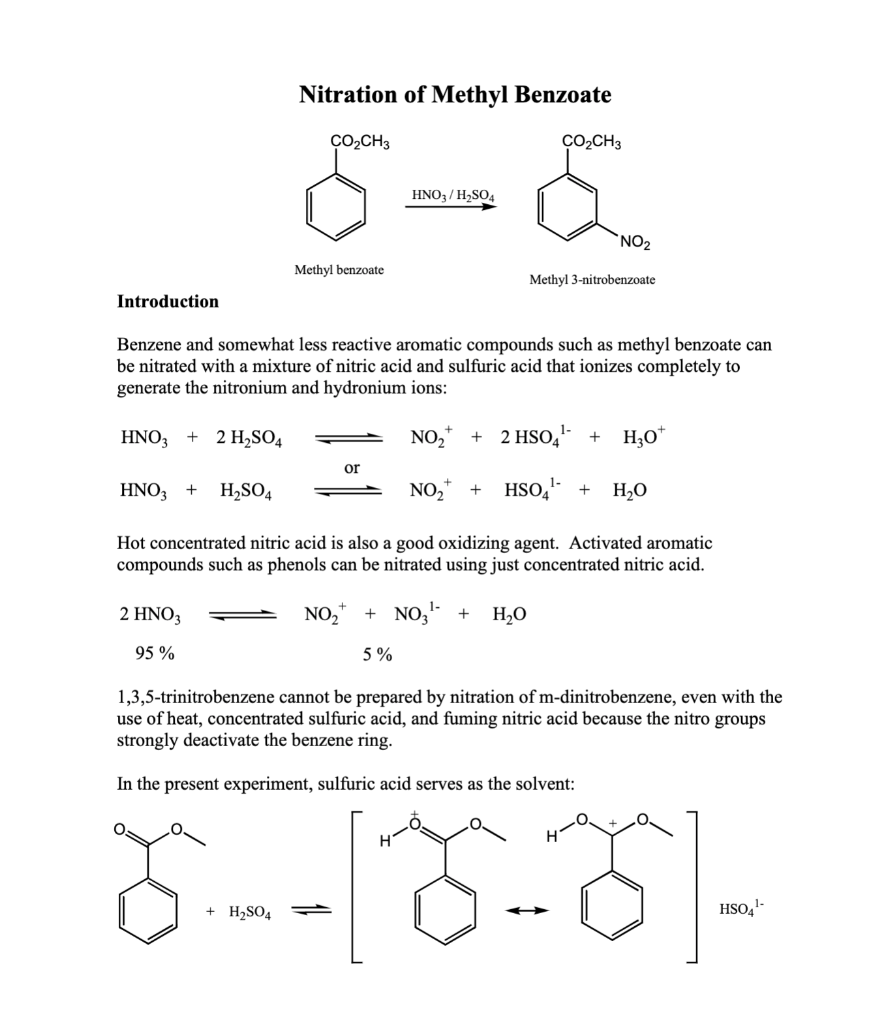

Please help. Thank you so much
Acquisition and Processing Parameters Acquisition Time: Nitration of Methyl Benzoate HNO3/H2SO4 Methyl benzoate Methyl 3-nitrobenzoate Introduction Benzene and somewhat less reactive aromatic compounds such as methyl benzoate can be nitrated with a mixture of nitric acid and sulfuric acid that ionizes completely to generate the nitronium and hydronium ions: HNO3+2H2SO4HNO3+H2SO4NO2++2HSO41+H3O+orNO2++HSO41+H2O Hot concentrated nitric acid is also a good oxidizing agent. Activated aromatic compounds such as phenols can be nitrated using just concentrated nitric acid. 2HNO3NO2++NO31+H2O95%5% 1,3,5-trinitrobenzene cannot be prepared by nitration of m-dinitrobenzene, even with the use of heat, concentrated sulfuric acid, and fuming nitric acid because the nitro groups strongly deactivate the benzene ring. In the present experiment, sulfuric acid serves as the solvent: +H2SO4 HSO41 Procedure Cool 2.4mL of concentrated sulfuric acid to 0C in a small round bottom flask and then add to it 1.2mL of methyl benzoate. Keep the mixture at 0C and add dropwise, using a Pasteur pipette, 2mL of a 50:50 mixture of concentrated sulfuric acid and concentrated nitric acid. Keep the reaction in the ice bath. Using a stirring rod, keep the reaction well mixed during the addition of the acids and do not allow the temperature of the mixture to rise above about 15C as judged by touching the reaction flask. After all the sulfuric aciditric acid mixture has been added, allow the mixture to warm to room temperature and after 15 minutes, pour it onto about 10g of ice in a small beaker. Isolate the solid product by suction filtration using the Hirsch funnel and filter flask. Wash the product well with cold water. Recrystallize the product from a minimum amount of hot methanol in a small beaker. This can be done by adding 10mL of methanol to the sample along with a boiling chip. Heat until boiling. If the product does not dissolve, add another 5mL portion of solvent and bring to a boil. Continue this until the product dissolves. Alternatively, the sample can be dissolved in a slightly larger quantity of hot methanol and while still hot, water can be added dropwise until cloudy. Slow cooling should produce crystals of methyl 3nitrobenzoate. Filter and wash the crystals with cold water. Allow the product to dry. Weigh your product, obtain an IR using a thin film or KBr pellet, obtain a 1HNMR in CDCl3, measure the melting point, and determine the percent yield. Melting point: 80C Questions: 1. Why does methyl benzoate dissolve in concentrated sulfuric acid? Write the equation showing the ions that are produced. 2. What would you expect the structure of the dinitro ester to be? Consider the direction effects of the ester and the first nitro group upon addition of the second nitro group. 3. Explain why methyl 4-nitrobenzoate does not form in this reaction. Use resonance structures to support your explanation. Summarize your results and analysis of the IR/NMR
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


