Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help!!! 6. The formation of the product actually goes through a unique electrophile (1Cl) and introduces a resonance structure in which all atoms have a
Help!!!
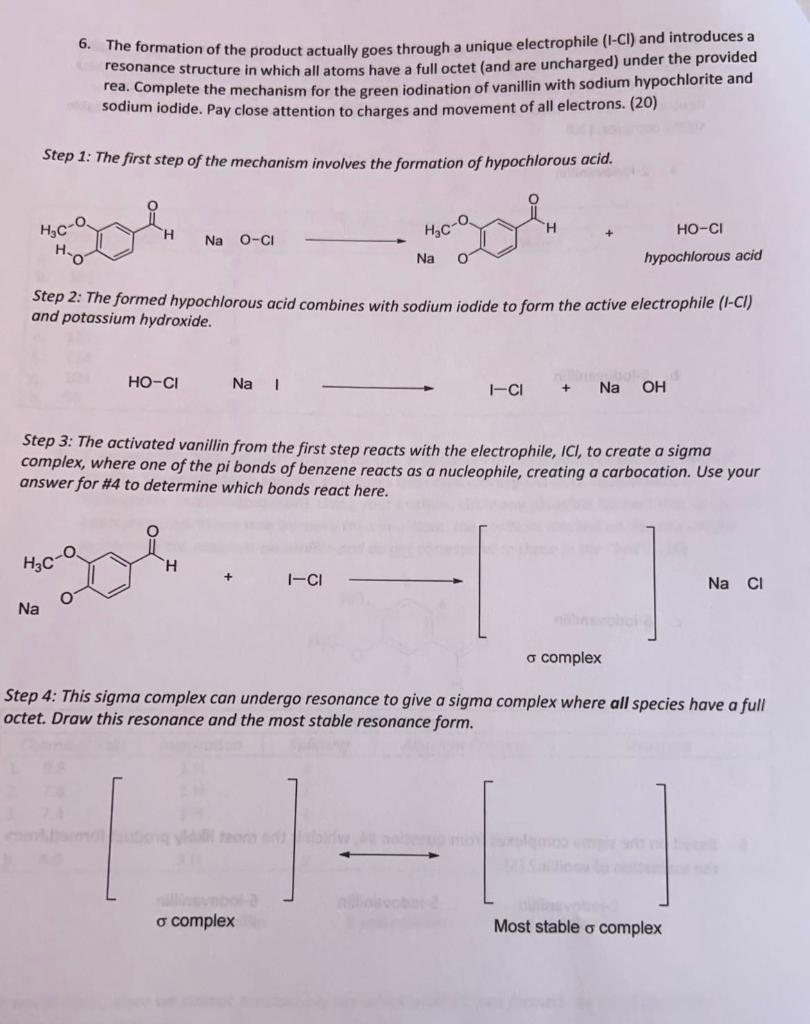
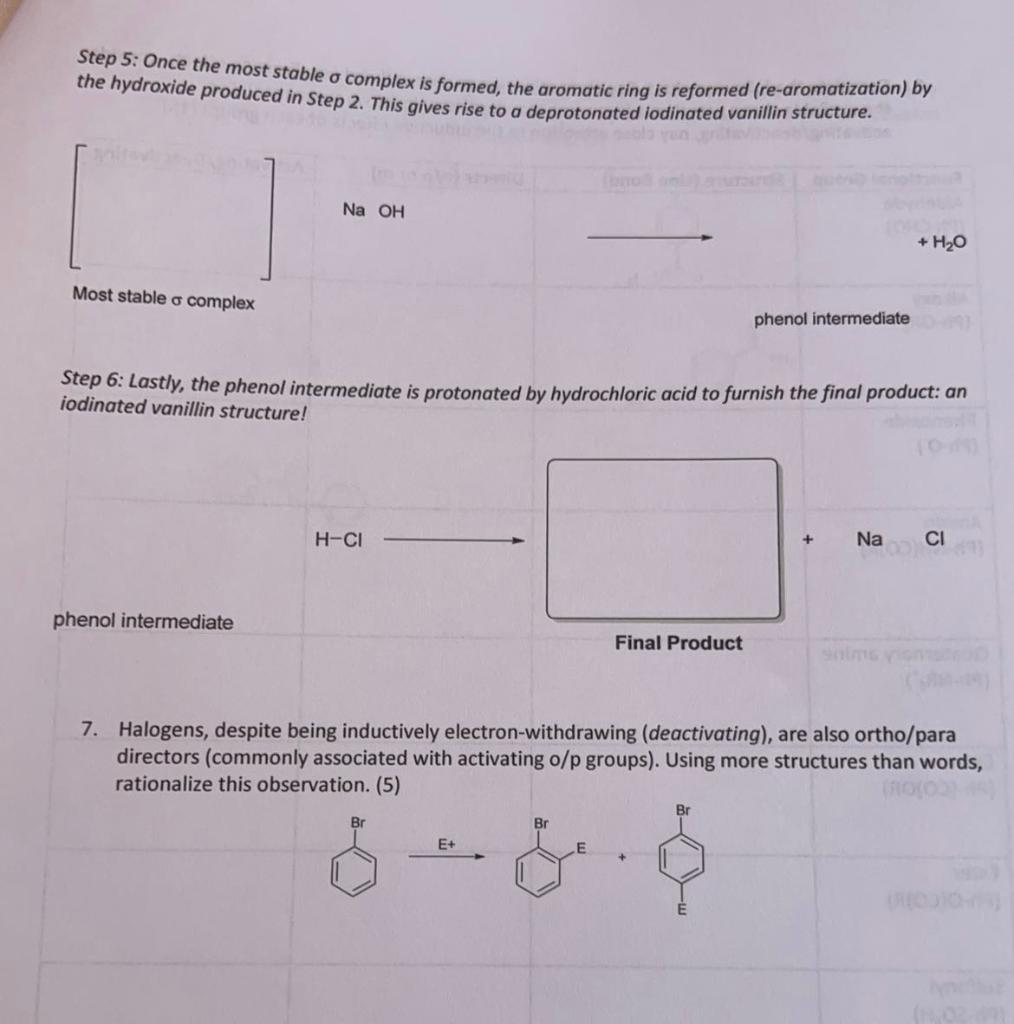
6. The formation of the product actually goes through a unique electrophile (1Cl) and introduces a resonance structure in which all atoms have a full octet (and are uncharged) under the provided rea. Complete the mechanism for the green iodination of vanillin with sodium hypochlorite and sodium iodide. Pay close attention to charges and movement of all electrons. (20) Step 1: The first step of the mechanism involves the formation of hypochlorous acid. NaOCl Step 2: The formed hypochlorous acid combines with sodium iodide to form the active electrophile (I-Cl) and potassium hydroxide. HOClNaIIl+NaOH Step 3: The activated vanillin from the first step reacts with the electrophile, ICI, to create a sigma complex, where one of the pi bonds of benzene reacts as a nucleophile, creating a carbocation. Use your answer for \#4 to determine which bonds react here. Step 4: This sigma complex can undergo resonance to give a sigma complex where all species have a full octet. Draw this resonance and the most stable resonance form. Step 5: Once the most stable complex is formed, the aromatic ring is reformed (re-aromatization) by the hydroxide produced in Step 2. This gives rise to a deprotonated iodinated vanillin structure. [1] Most stable complex phenol intermediate Step 6: Lastly, the phenol intermediate is protonated by hydrochloric acid to furnish the final product: an iodinated vanillin structure! phenol intermediate Final Product 7. Halogens, despite being inductively electron-withdrawing (deactivating), are also ortho/para directors (commonly associated with activating o/p groups). Using more structures than words, rationalize this observation. (5) E+Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


