Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest
help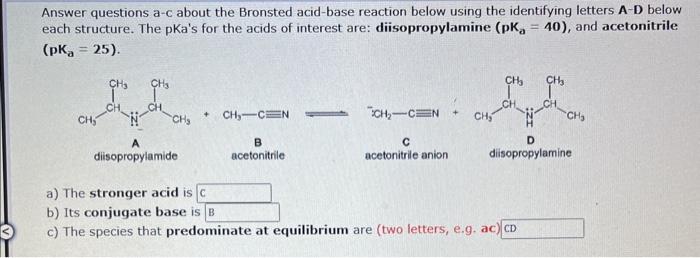
Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: diisopropylamine (pKa=40), and acetonitrile (pKa=25) +CH3CN iCH2CN disopropylamide B atonitrile acetonitrile anion disopropylamine a) The stronger acid is b) Its conjugate base is c) The species that predominate at equilibrium are (two letters, e.g.ac) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


