Answered step by step
Verified Expert Solution
Question
1 Approved Answer
HELP ASAP 9b0699=Aeynujog+XV=llando0L7UaaNEM Pre-10is Calculations: flask: v1=5.00mv2v2=m1v2M2=? Diasic 3: H2=50.003.00.00150= flosic 2m2=5002.00.00000=7.2103 Finsic n) m2=50.04.00.0460=1.441041/1 Fiask 5. 2=5.00.00.0050=216101 Calculations 1. All calculations needed to make
HELP ASAP
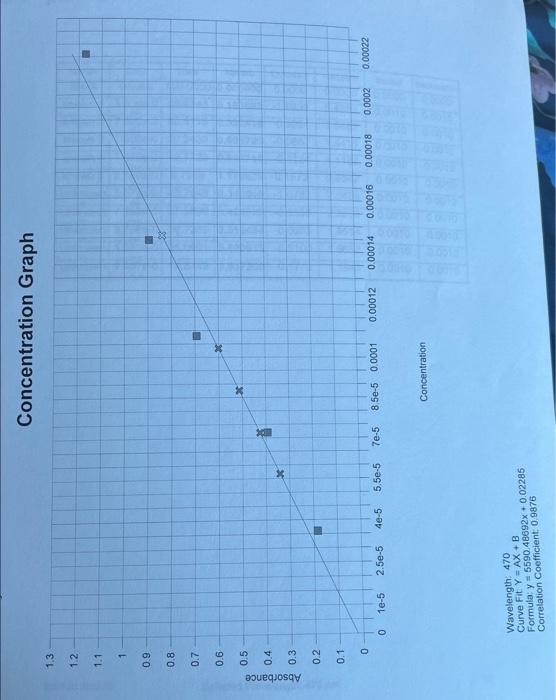
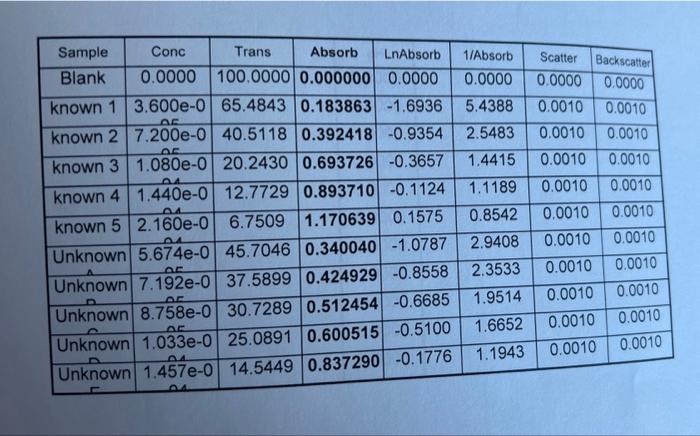
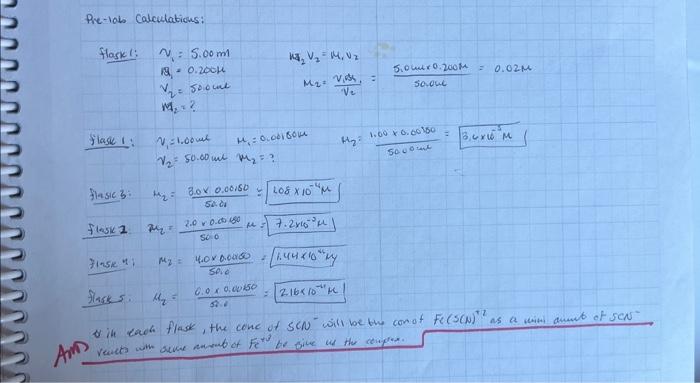

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


