Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help fill in the blanks please temperature. Continue recording the temperature every 30 seconds for 5 minutes. Stop only when a constant decrease in temperature
Help fill in the blanks please 
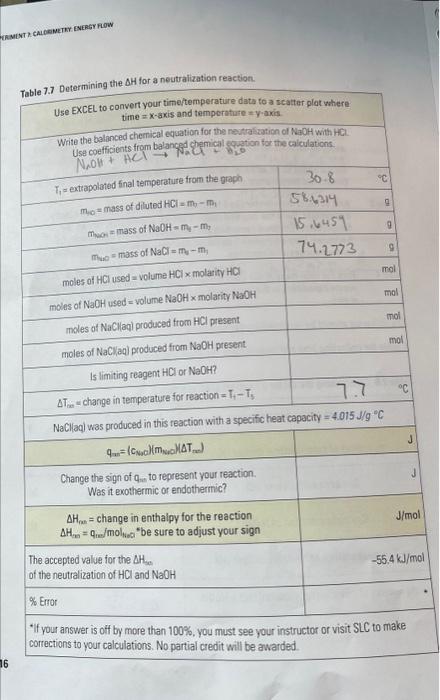
temperature. Continue recording the temperature every 30 seconds for 5 minutes. Stop only when a constant decrease in temperature is noted Step 4 Find the 29.9C30.0C29.9C29.8C29.7C29C2 for four consecutive readings. waste containers of the calorimeter (m8). Discard the solution in designated water. The following should be recorded in your notebook: A table for collecting time and temperature readings and Table 7.6 and Table 7.7. Table 7.6 Determining the H for a neutralization reaction. \begin{tabular}{|c|c|} \hlinem1= mass of empty calorimeter & 6.3382 \\ \hlinem7= mass of calorimeter + diluted HCl & 3,96 \\ \hline C1= concentration of HCl & 3.0 \\ \hline C2= concentration of NaOH & 3.0 \\ \hline T5= temperature of NaOH or diluted HCl & 23.1 \\ \hline m8= mass of calorimeter + diluted HCl+NaOH & 80.65 \\ \hline \end{tabular} Table 7.7 Determining the OH for a neutraiization reaction. Use EXCEL to convert your time/temperature data to a scalter plat where time =x-axis and temperature = y-axis. Write the balanced chernical equation for the nevtraf ation of NaOH with HCL. - If your answer is off by more than 100%, you must see your instructor or visit SLC to make corrections to your calculations. No partial credit will be awarded 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


