Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help! I Review Constants Part A How many moles of O, are needed to react with 1.55 mol of CH? Express your answer to three
help! 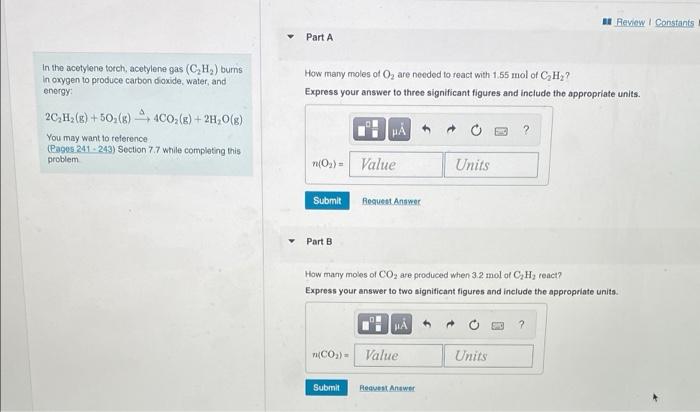
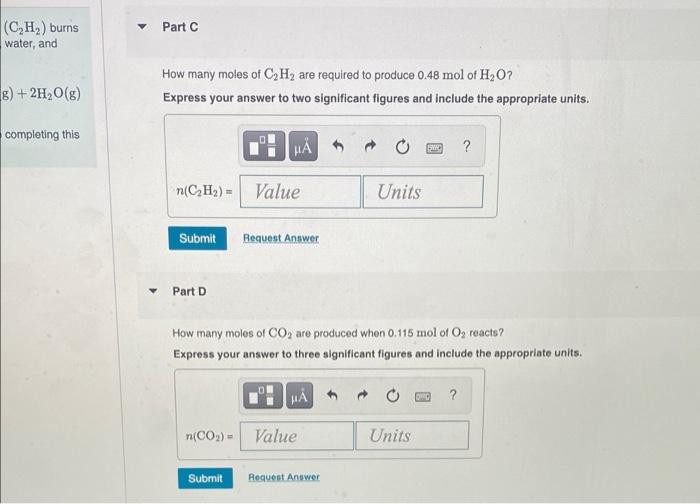
I Review Constants Part A How many moles of O, are needed to react with 1.55 mol of CH? Express your answer to three significant figures and include the appropriate units. In the acetylene torch, acetylene gas (C,H,) burns In oxygen to produce carbon dioxide, water, and energy 2CH2(8) +50,(6) 400(E)+24,0(e) You may want to reference (Pages 241 243) Section 77 while completing this problem H (Oy)= Value Units Submit Request Answer Part B How many moles of CO2 are produced when 3.2 mol of C, H, react? Express your answer to two significant figures and include the appropriate units. MA ? (CO) - Value Units Submit Peavest Answer Part C (C,H,) burns water, and 8) + 2H2O(8) How many moles of C2H2 are required to produce 0.48 mol of H2O? Express your answer to two significant figures and include the appropriate units. completing this H ? r(CH) - Value Units Submit Request Answer Part D How many moles of CO2 are produced when 0.115 mol of O, reacts ? Express your answer to three significant figures and include the appropriate units. ? n(CO2) - Value Units Submit Request 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


