Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help me find out the answers Pls (a) Air (B) 2 23-24 1 Liquid Benzene (A) Figure 1 Diffusion of benzene through stagnant, nondiffusing air
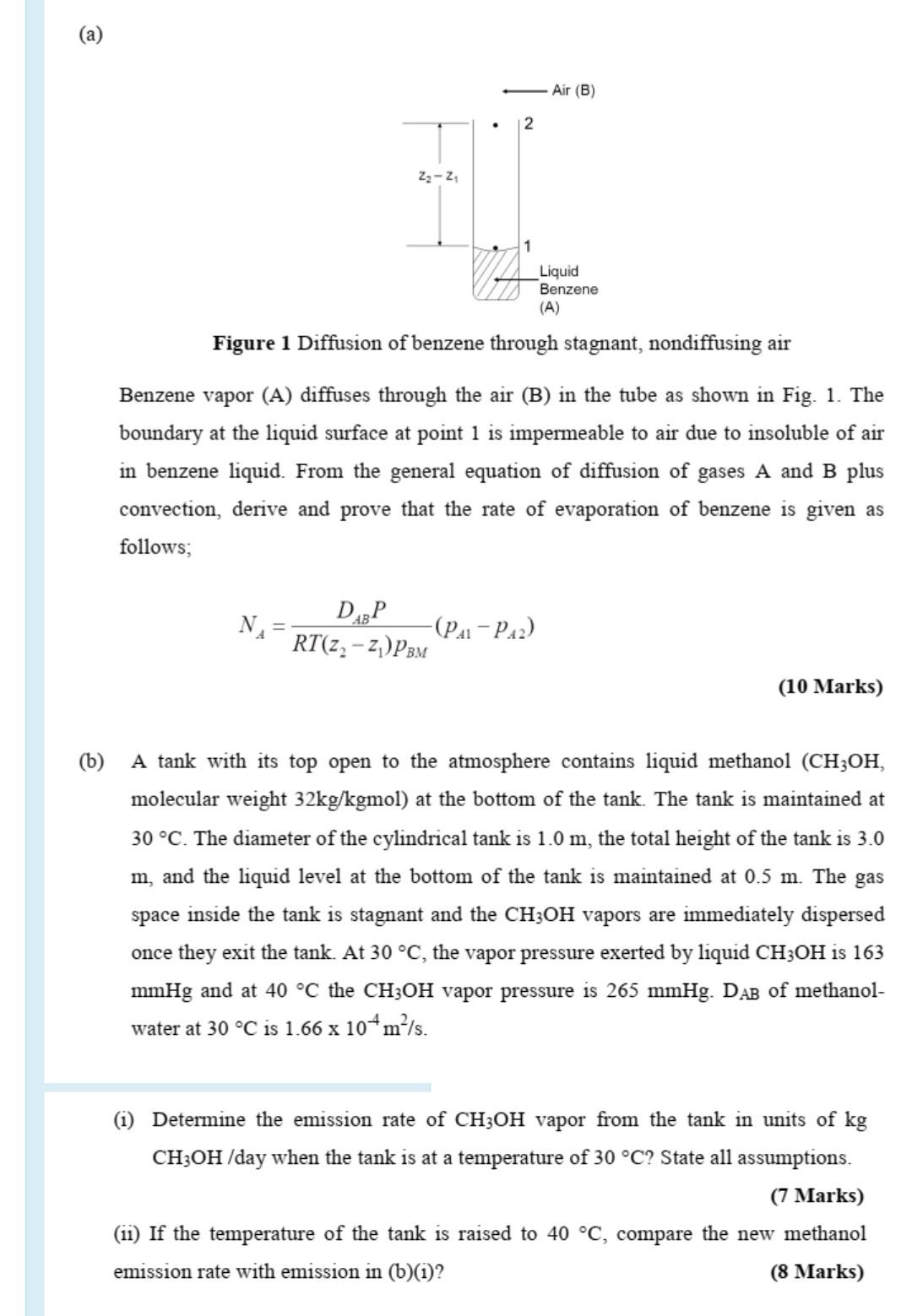
Help me find out the answers Pls
(a) Air (B) 2 23-24 1 Liquid Benzene (A) Figure 1 Diffusion of benzene through stagnant, nondiffusing air Benzene vapor (A) diffuses through the air (B) in the tube as shown in Fig. 1. The boundary at the liquid surface at point 1 is impermeable to air due to insoluble of air in benzene liquid. From the general equation of diffusion of gases A and B plus convection, derive and prove that the rate of evaporation of benzene is given as follows; NA DABP -(PA-PA2) RT(22 - 2)PBM (10 Marks) (b) A tank with its top open to the atmosphere contains liquid methanol (CH3OH, molecular weight 32kg/kgmol) at the bottom of the tank. The tank is maintained at 30 C. The diameter of the cylindrical tank is 1.0 m, the total height of the tank is 3.0 m, and the liquid level at the bottom of the tank is maintained at 0.5 m. The gas space inside the tank is stagnant and the CH3OH vapors are immediately dispersed once they exit the tank. At 30 C, the vapor pressure exerted by liquid CH3OH is 163 mmHg and at 40 C the CH3OH vapor pressure is 265 mmHg. DAB of methanol- water at 30 C is 1.66 x 104m/s. (1) Determine the emission rate of CH3OH vapor from the tank in units of kg CH3OH /day when the tank is at a temperature of 30 C? State all assumptions. (7 Marks) (11) If the temperature of the tank is raised to 40 C, compare the new methanol emission rate with emission in (b)(1)? (8 Marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


