Answered step by step
Verified Expert Solution
Question
1 Approved Answer
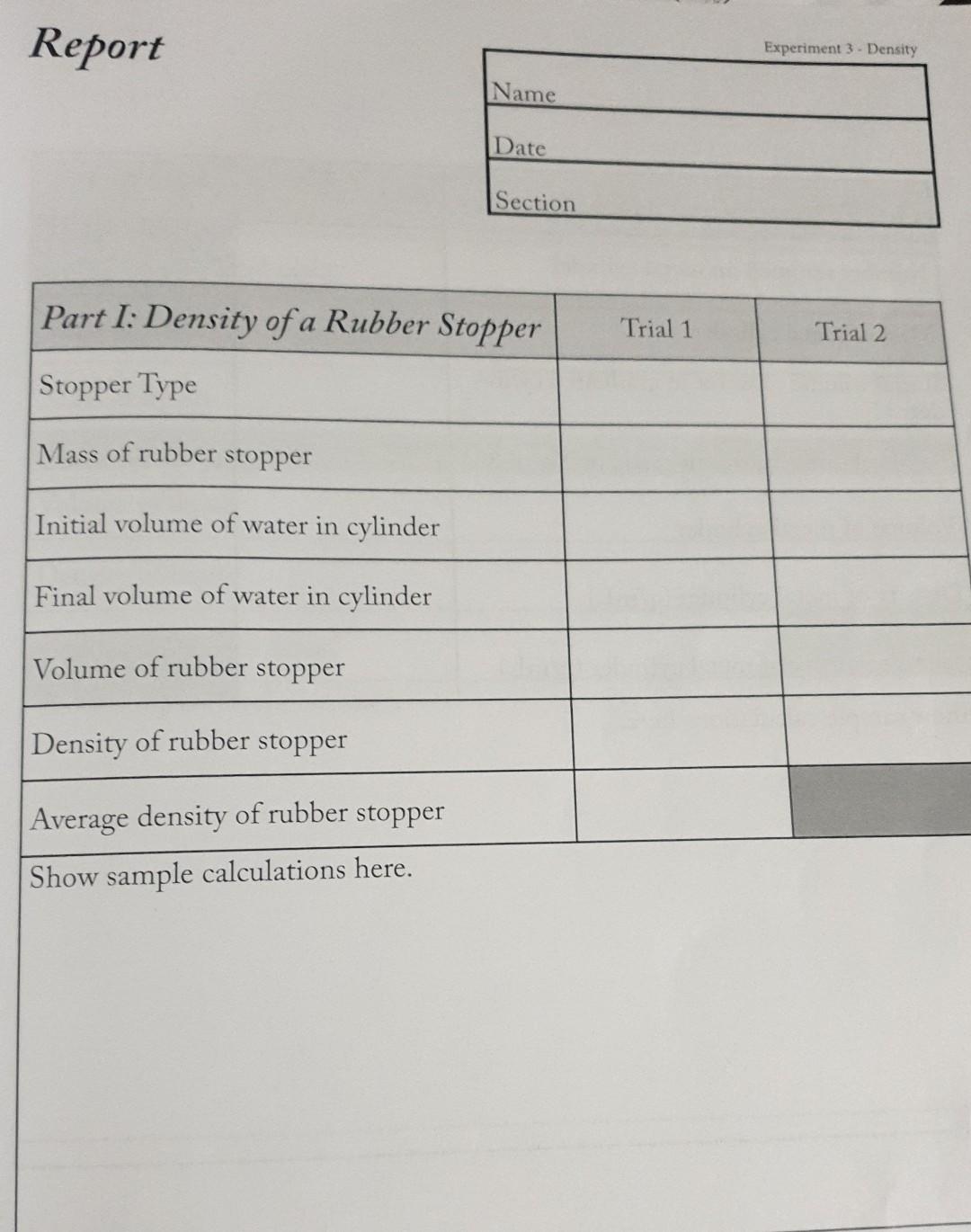
help me please Report Name Date Section begin{tabular}{|l|l|l|} hline Part I: Density of a Rubber Stopper & Trial 1 & Trial 2 hline Stopper




help me please
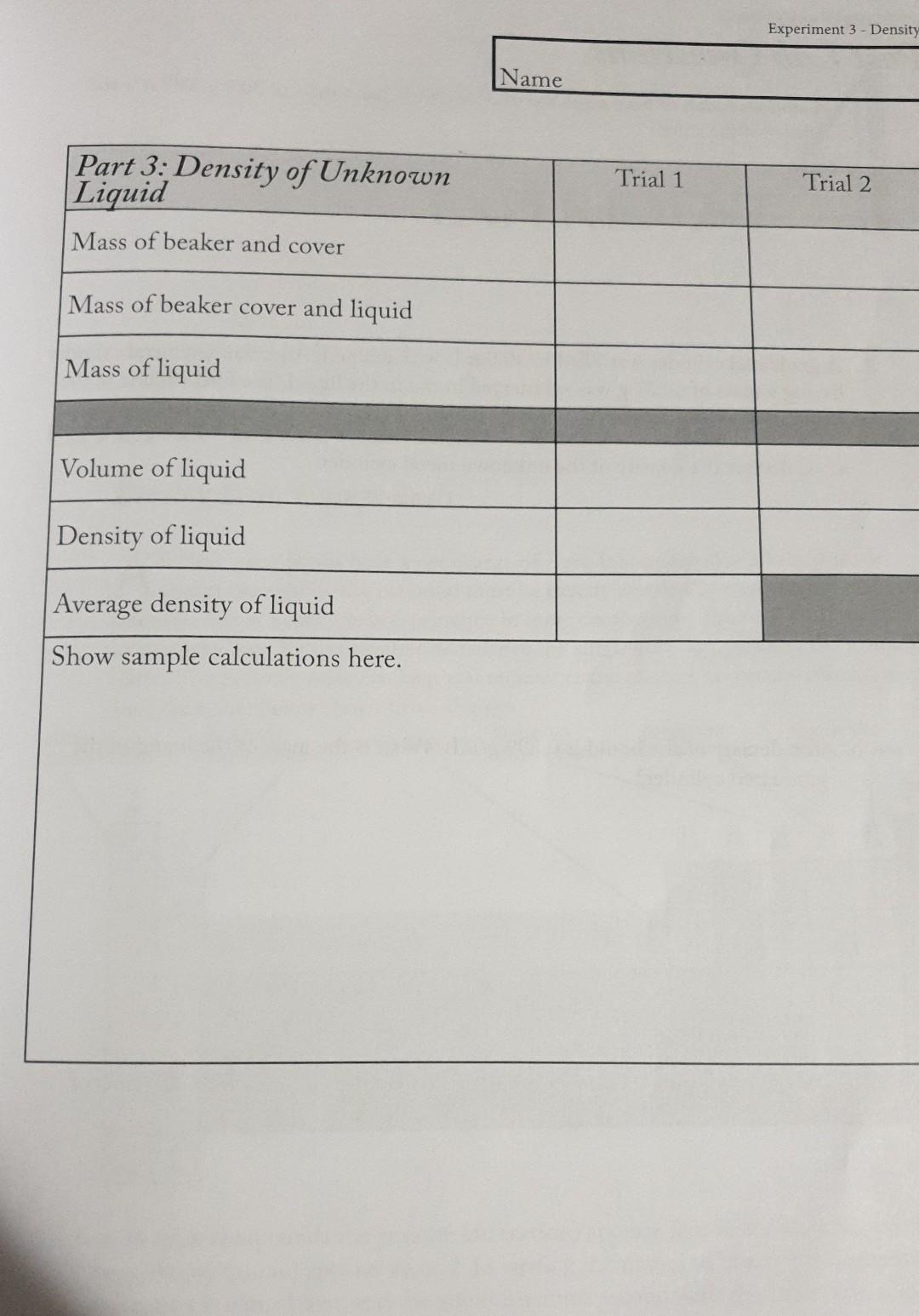
Report Name Date Section \begin{tabular}{|l|l|l|} \hline Part I: Density of a Rubber Stopper & Trial 1 & Trial 2 \\ \hline Stopper Type & & \\ \hline Mass of rubber stopper & & \\ \hline Initial volume of water in cylinder & & \\ \hline Final volume of water in cylinder & & \\ \hline Volume of rubber stopper & & \\ \hline Density of rubber stopper & & \\ \hline Average density of rubber stopper & & \\ \hline \end{tabular} Show sample calculations here. \begin{tabular}{|l|l|l|l|} \hline Part 2: Density of a Metal Cylinder & & Trial & \\ \hline Number stamped on metal cylinder & & & \\ \hline Mass of metal cylinder & & \\ \hline Initialvolumeofwateringraduatedcylin-der & & \\ \hline Final volume of water in graduated cylinder & & \\ \hline Volume of metal cylinder & & \\ \hline Density of metal cylinder (g/mL) & & \\ \hline Average density of metal cylinder (g/mL) & & \\ \hline Show sample calculations here. & & \\ \hline \end{tabular} Name \begin{tabular}{|l|l|l|} \hline Part3:DensityofUnknownLiquid & Trial 1 & Trial 2 \\ \hline Mass of beaker and cover & & \\ \hline Mass of beaker cover and liquid & & \\ \hline Mass of liquid & & \\ \hline & & \\ \hline Volume of liquid & & \\ \hline Density of liquid & & \\ \hline Average density of liquid & & \\ \hline \end{tabular} Show sample calculations here. Post Lab Questions 1. A stone is found to have a volume of 34.9cm3. It has a mass of 30.8g. What is the density of the stone? 2. A graduated cylinder was filled to 30.0mL with a liquid. An unknown metal cylinder having a mass of 60.27g was submerged in the in the liquid. The final volume of the liquid level was 45.2mL. a. Calculate the density of the unknown metal cylinder. b. The density of the liquid is 0.899g/mL. What is the mass of the liquid in the graduated cylinder? 3. The density of gasoline is 0.6775g/cm3. Calculate the volume of 200.0 grams of gasoline. Balance, graduated cylinder, beaker, watch glass, small rubber stoppers, metal cylinder and unknown liquid. Introduction The density of a substance is a physical property that requires measurements of mass d=m/V. Using the metric system, the ratio for volume is density, d and is written as g/mL. Since the density of gases is much less than the densities of liquids and solids, the density of gases is usually expressed in grams per liter, g/L. Matter expands and contracts according to temperature. For liquids and solids, the change in volume is very small, but for gases, a temperature change results in a proportional change in the volume. Therefore, gas density is dependent upon temperature; for liquids and solids, the small effect on volume is often ignored. It is important to understand how density plays a role in everyday life. For example, wine makers measure densities to determine sugar content in the fermentation process and auto mechanics measure the density of the acid in car batteries to determine charge. Procedure 11 weighings and volumes should be read to the highest precision. This is usually 0.1mL Avolume, and 0.1g to 0.001g for mass. The precision to which you measure mass depend on the type of balance called for in the experiment. Follow all safety procedures throughout experiment. Experiment 3-Density Part 1: Density of a Rubber Stopper Obtain a small rubber stopper, it must be small enough to fit inside a 50mL graduated cylinder. Weigh and record the mass of the dry stopper. Use tap water to fill your graduated cylinder to approximately 25mL. Read and record this volume to the nearest 0.1mL remembering to read the volume at the bottom of the meniscus. Carefully submerge the rubber stopper in the graduated cylinder. Read and record the new volume. What is the volume of the rubber stopper? Show your calculation. What is the density of the rubber stopper? Once again, show your calculation. Use the graduated cylinder containing the water and rubber stopper for Part 2. Part 2: Density of a Metal Cylinder Obtain a metal cylinder from your instructor and record the cylinder number on your data sheet. Read and record the mass of the metal cylinder. Record the initial volume of water and stopper in the graduated cylinder (it may be the final volume of water and stopper from Part 1). Slightly tilt the graduated cylinder containing the tap water and rubber stopper. Carefully slide and submerge your metal cylinder. The rubber stopper will act as a bumper and prevent the graduated cylinder from breaking. Read and record the new volume. Return the metal cylinder. Calculate the volume and density of the metal cylinder. Show your calculations. Part 3: Density of an unknown liquid Weigh and record the mass of a clean dry 150mL beaker and watch-glass cover to the highest precision of the balance. Place about 2535mL of the unknown liquid in a 50 mL graduated cylinder. Read and record the volume to the nearest 0.1 milliliter. Carefully transfer as much liquid as possible to the clean, dry 150mL beaker, cover with the watch glass and reweigh. Repeat the procedure with a different volume of the same liquid. Cal culate the density of your unknown liquid for each trial. Be sure to show your calculations. Take an average of the two density determinations that you madeStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


