Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help please In the reaction shown below. trifluoroacetic acid protonates water to make trifluoroacetate anion and hydronium ac :0: HO + H 'H pK, 0.23
help please 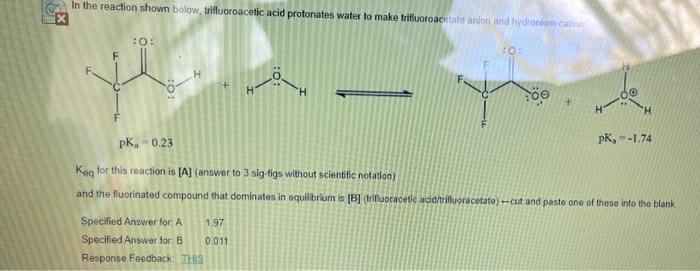
In the reaction shown below. trifluoroacetic acid protonates water to make trifluoroacetate anion and hydronium ac :0: HO + H 'H pK, 0.23 pK, -1.74 Keg for this reaction is [A] (answer to 3 sig-tigs without scientific notation) and the fluorinated compound that dominates in equilibrium is [B] (trifluoracetic acid trifluoracetato) --cut and paste one of these into the blank Specified Answer for A 1.97 Specified Answer for B 0.011 Response Foedback: THIS 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


