Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help, please The molar absorptivities of compounds A and B were measured with pure samples of each: A mixture of the compounds X and Y
Help, please 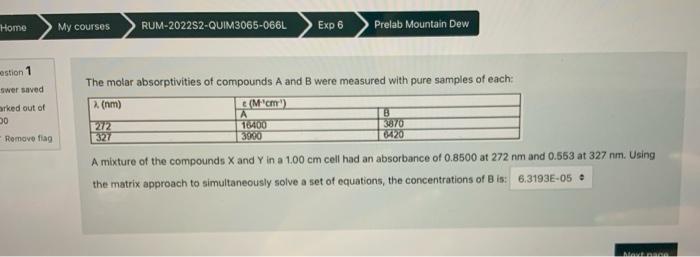
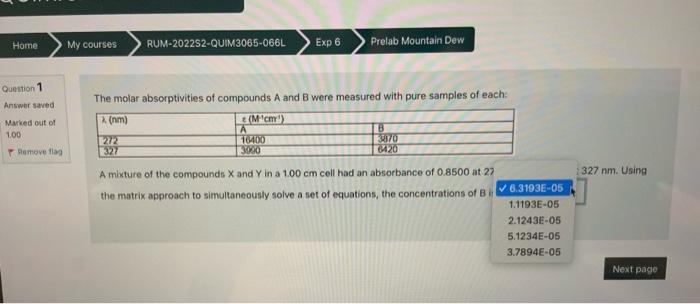
The molar absorptivities of compounds A and B were measured with pure samples of each: A mixture of the compounds X and Y in a 1.00cm cell had an absorbance of 0.8500 at 272nm and 0.553 at 327nm. Using the matrix approach to simultaneously solve a set of equations, the concentrations of B is: Quettion 1 Answer saved The molar absorptivities of compounds A and B were measured with pure samples of each: Marked out of 1.00 Gemove ila? A mixture of the compounds X and Y in a. 1.00 cm cell had an absorbance of 0.8500 at 27 the matrix approach to simultaneously solve a set of equations, the concentrations of B 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


