Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help plz 9.3 Lattice Energy Draw Born-Haber cycle for the formation of sodium chloride. 9.3 Lattice Energy Use the following data to calculate the lattice
Help plz




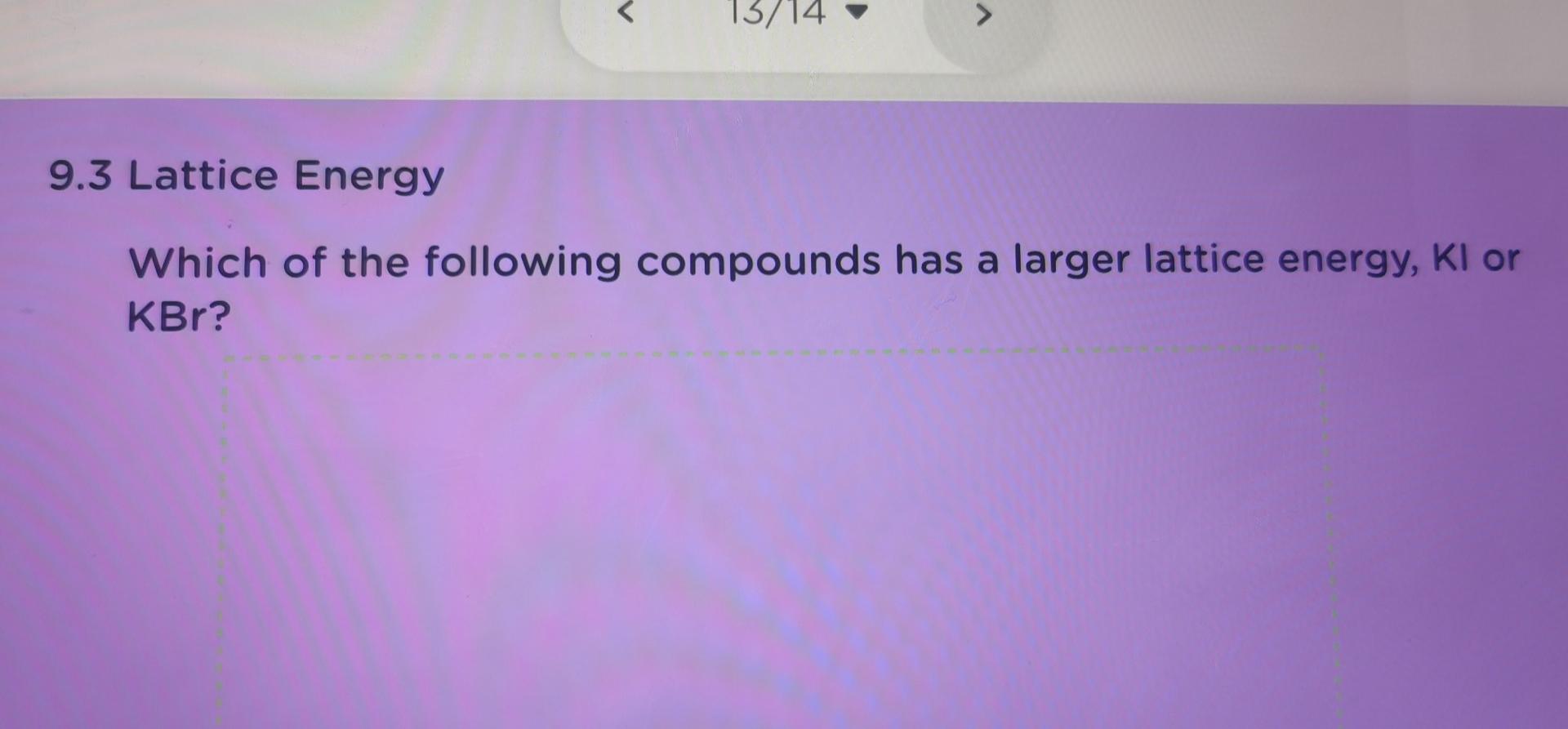
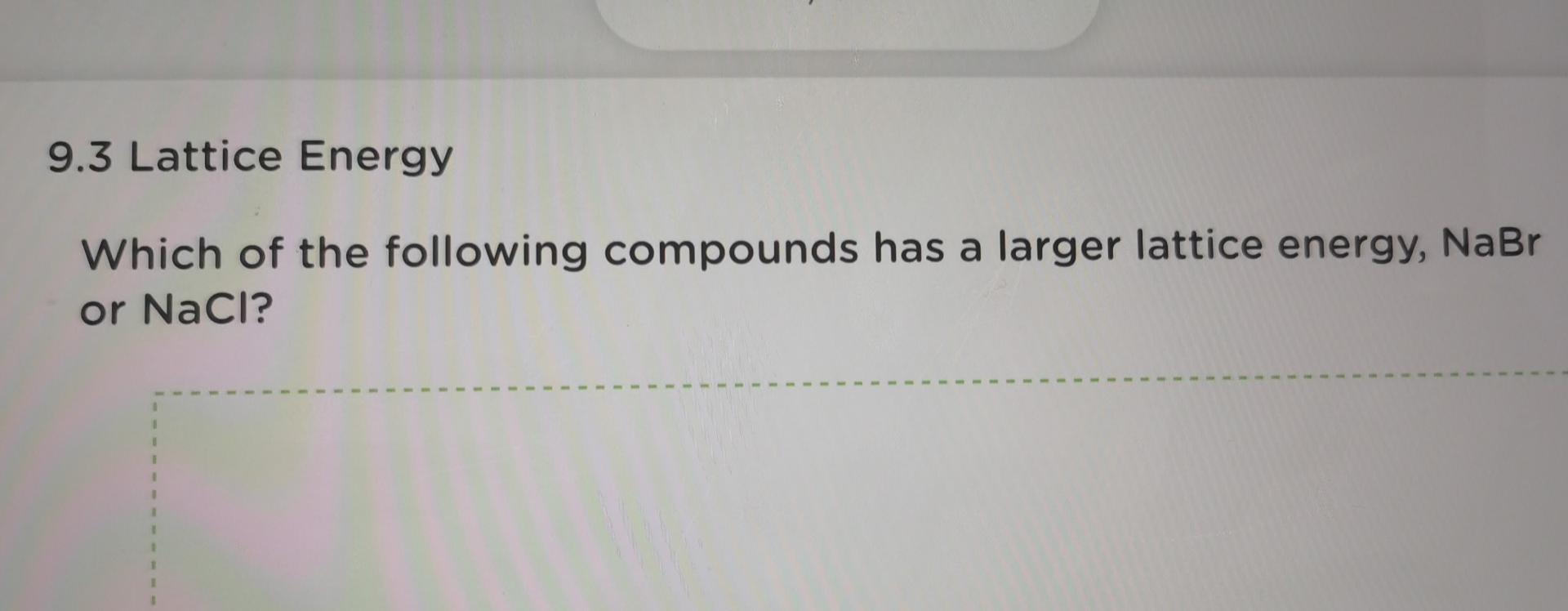
9.3 Lattice Energy Draw Born-Haber cycle for the formation of sodium chloride. 9.3 Lattice Energy Use the following data to calculate the lattice energy of sodium chlor You must write all thermochemical equations for the steps of the cycle. 1 AHI - heat of formation of sodium chloride 1.411.3 kJ molt 2 AH1 - heat of sublimation of Na(s) 108.7 kJ molt AH2 =lonization energy of Nalo) 496 kJ molt AH3 - dissociation energy of C12) 244 kJ molt 5 AHA - Electron affinity of Cl(a) . 349 KJ molt U-lattice energy of Nacho KJ molt Lattice Energy ange the compounds NaF, MgO, and AIN in order of increasing latt ergy. 9.3 Lattice Energy Which of the following compounds has a larger lattice energy, Na20 or MgO? 13/14 9.3 Lattice Energy Which of the following compounds has a larger lattice energy, Kl or KBr? 9.3 Lattice Energy Which of the following compounds has a larger lattice energy, NaBr or NaCl? 1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


